
Efficacy of MR-guided focused ultrasound surgery (MRgFUS) of uterine fibroids: evaluation of non perfused volume (NPV), fibroid shrinkage and clinical improvement at 6 months follow-up
Introduction
Uterine fibroids (myomas or leiomyomas) are the most common benign tumours in the female genital tract, arising from the smooth-muscle cells of human uterus (1). Almost 30% of women in reproductive age are affected by uterine leiomyomas, especially from the age of 35 to the menopause (2,3), and nearly 30% of women with fibroids have symptoms like uterine bleeding and iron deficiency anaemia, pelvic pressure, and reproductive dysfunction (2,3).
There are many treatment modalities for symptomatic fibroids. The most common are hysterectomy and myomectomy, invasive surgical procedures associated with surgical risks and complications, that require 3-4 days hospitalization and many weeks of patient recovery (2,4).
Actually alternative and less invasive treatments have been introduced to decrease the perioperative morbidity and to reduce healthcare costs. These are uterine artery embolization (UAE) and high intensity focused ultrasound (HIFU) guided by sonography or guided by magnetic resonance-guided focused ultrasound surgery (MRgFUS).
MRgFUS in a non invasive treatment that combines a HIFU beam with the magnetic resonance imaging (MRI) properties of anatomic resolution and thermal imaging. The beam causes an increase in the temperature of the targeted tissue (till 70-80 °C) and then a coagulative necrosis, identified with the non perfused volume (NPV) (tissue without blood flow evaluated at the end of the treatment with contrast media) (1,2). MR images enable to define the target of treatment and the structures to be avoided, such as small bowel, and permit a real-time monitoring of temperature and localization of the tissue being treated (3-5).
This treatment is well stand by patients, it has low risk of complications and avoids radiation exposure; there is no hospitalization and patient recovery time is minimal (24-48 hours vs. 5 weeks for hysterectomy) (6).
The aim of this prospective study was to determine the efficacy of MRgFUS treatment of uterine fibroids in symptomatic women, evaluating fibroid shrinkage and clinical improvement after 6 months; and to assess if the NPV measured immediately after treatment could be predictive of the efficacy of the treatment.
Methods
All the MRgFUS treatments were performed in our hospital between September 2010 and October 2012, after receiving approval by institutional review boards. All patients gave informed consent for MRgFUS treatment.
We used the ExAblate 2000 system (InSightec, Haifa, Israel), which consist of a phased array transducer (208 elements, 0.95-1.35 MHz), a computer-controlled positioning system, a multichannel radiofrequency amplifier and a user interface (7). These components operate in conjunction with a 1.5 T MRI unit (Signa, GE Medical Systems, Milwaukee, WI).
Patient selection
All women were older than 18 years, with clinically symptomatic uterine fibroids and no other major medical disease.
For inclusion in our study women should have no more than three symptomatic fibroids, with a diameter between 3 and 10 cm and a hypontense signal on T2 weighted images; fibroids with high T2 signal intensity were excluded because these have been found to be less responsive to MRgFUS treatment (8,9).
Exclusion criteria included absolute contraindications and relative contraindications. Absolute contraindications are positive pregnancy test on the day of treatment, contraindications to MRI (e.g., pacemakers) and peduncolated fibroids with a narrow stalk. Relative contraindications include fibroids with a diameter greater than 10 cm, that may benefit from gonadotropin-releasing hormone (GnRH) agonist pre-treatment to shrink (10,11) and thick abdominal scars that could absorb energy and determine skin burn (12). Even posterior fibroids and the presence of bowel interposed between abdominal wall and the fibroid to be treated are relative contraindications, but in the most instances we could treat the patients after a combination of rectal filling (with US gel) and bladder filling (with saline) (12,13).
All the candidates to treatment underwent an MRI exam with Gadolinium contrast agent and a consult with gynecologist and radiologist to evaluate the feasibility of MRgFUS.
Patient preparation
Patient was informed not to eat during the previous 6 hours and to shave the area between umbilicus and pubic bone because the depilation is very important for not increase temperature, preventing burn skin.
The presence of scar is evaluated before the treatment with the MR imaging; the hypertrophic scar excludes the patient from treatment because it can accumulate temperature and deviate the US beam.
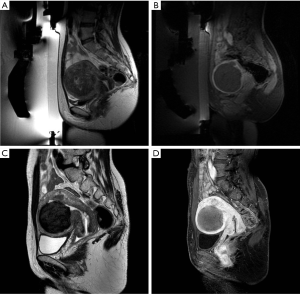
Immediately before treatment the patient was prepared with the introduction of IV line and Foley catheter in the bladder and then she was positioned prone on a special MRI table containing the transducer. Multiplanar turbo spin echo (TSE) T2 weighted MRI acquisitions of the pelvic region (Table 1) were obtained so the fibroid was placed directly over the transducer. On these images we defined the region of treatment (ROT) on the targeted fibroid and the structures to preserve (small bowel, pubic bone, skin and sacral nerve). If needed bladder was filled with saline and rectus with US gel to displace the uterus anteriorly.
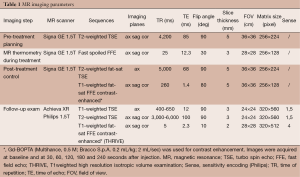
Full table
During all treatment the patient had a noninvasive cardiorespiratory monitoring and a conscious sedation with continuous infusion of remifentanyl (Ultiva, GlaxoSmithKline, UK) (0.045-0.055 gamma/Kg/min) was administered, to allow communication with the treating team.
MRgFUS treatment
The system automatically plans the treatment computing the optimal sonication grid necessary to coagulate the targeted volume. The US beam causes an increase in the temperature of every single spot of sonication reaching 70-80 °C, so coagulative tissue necrosis is obtained. Each sonication lasts approximately 20-30 seconds and it is followed by a cooling period of 60-90 seconds necessary for the skin to return to baseline temperature. During each sonication real time gradient echo images are obtained (Table 1) to evaluate the expected path of the US beam and the anatomy of the treated fibroid. MR thermometry measures the temperature in the targeted area generating a temperature map. The duration of the treatment depends on the number of sonications necessary to cover all the ROT.
Immediately at the end of the treatment an MRI exam with intravenous gadolinium contrast agent administration was performed (Multihance, Bracco SpA): we acquired multiplanar T2 weighted and T1 weighted images and a dynamic study with contrast-enhanced fat-suppressed images to quantify the tissue without blood flow (NPV) of the fibroid, corresponding to the treated necrotic area.
An MRI exam with T1 weighted and T2 weighted images and a dynamic study was performed 6±1 months after treatment to evaluate the fibroid shrinkage and the residual NPV.
Volume measurement
For the measurements of the different volumes we used the Osirix 4.3 software, drawing on each slice the single region of interest (ROI), always on sagittal plane, and computing the volume with the three dimensional (3D) reconstruction. The initial fibroid volume was measured on pre-treatment T2 weighted images, the post-treatment NPV on the late phase of the contrast enhanced study performed immediately after treatment; the 6-month follow-up fibroid volume was measured on T2 weighted images of the follow-up exam and the residual NPV on the late phase of the contrast enhanced study performed after 6 months. Then post-treatment NPV was compared to initial fibroid volume, and was expressed as a percentage, termed post treatment NPV ratio. Fibroid shrinkage after 6 months was calculated by subtracting 6-month fibroid volume from the initial fibroid volume, and dividing the result by the initial fibroid volume.
Clinical evaluation
Patients were asked to complete a uterine fibroid symptoms and quality of life (UFS-QOL) questionnaire at baseline and at follow-up. This questionnaire is specific to uterine fibroids and their treatment: it is composed of 37 items and responses for each item were scored from 1 (no symptoms) to 5 (major symptoms). Of all these, eight items are related to the symptom severity score (SSS) and 29 items are related to the quality of life score (QOLS). The sums of the two different scores were transformed into a 0-100 scale for comparison (14,15). For the SSS higher scores indicate worse symptoms. For the QOLS higher scores indicate better quality of life.
The SSS and QOLS scores were calculated for each patient and then measured values were expressed in mean ± standard deviation (SD).
Statistical analysis
Measured values were displayed as mean ± SD.
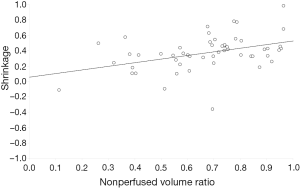
The statistical significance was evaluated using Student’s t-test and Wilcoxon sum ranked test for the comparison between SSS and QOLS values at baseline and at follow-up. Linear regression analysis was used to evaluate the correlation between fibroid shrinkage after 6 months and post treatment NPV ratio (Pearson’s r).
P value of less than 0.05 was considered to indicate a significant difference.
Results
A total of 72 symptomatic uterine fibroids in 60 women (range, 35-54 years old; average, 44 years old) were treated with MRgFUS between September 2010 and October 2012. Fifty-one patients were treated for a single fibroid, six patients for two fibroids and three patients for three fibroids simultaneously. Only one patient underwent two treatments for two different fibroids, the second 10 months after the first (Table 2). No significant complications were reported, only a minor skin burn that resolved with topical cream.
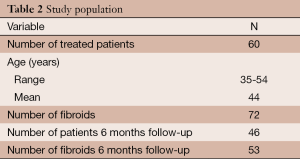
Full table
The mean pre-treatment fibroid volume was 134±139 cm3 (range, 4.4-587 cm3). Immediately after treatment the mean NPV of the treated fibroids was 67%±20% of initial volume.
Between March 2011 and October 2012, a total of 53 fibroids in 46 women underwent a follow-up MRI exam with gadolinium contrast agent 6±1 months after treatment.
Of the other six patients due for the 6-month follow-up exam by October 2012, three patients had hysterectomy because they still have symptoms even after MRgFUS treatment, one patient had an allergic reaction to the contrast media at the follow-up exam and was excluded from the study, and two patients did not want to come to undergo the exam.
Six months after treatment the treated fibroids average volume significantly decreased from 134±139 to 96±116 cm3 (P=0.0001) with an average volume reduction of 37%±22% (Table 3) (Figure 1).
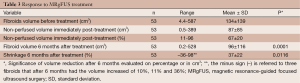
Full table
A linear regression analysis showed a significant correlation between post-treatment NPV ratio and fibroid shrinkage after 6 months (P=0.0116) (Figure 2). So the larger is the NPV after treatment, the greater is the fibroid shrinkage after 6 months.
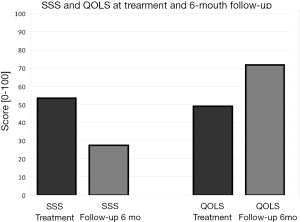
The mean SSS value at baseline was 53±23 (n=38) and after 6 months it significantly decreased to 27±17 (P<0.001), with an important symptoms reduction. Similarly the mean QOLS value before treatment was 49±24 (n=38) and it significantly increased to 72±21 (P<0.001), with an improvement in the quality of life of the patients (Figure 3).
Discussion
Uterine fibroids are a significant healthcare concern for many women in reproductive age. The causes of fibroids are unknown, however, genetic and hormonal factors have an essential role in their development. Fibroids are symptomatic in approximately 30% of women of childbearing age; symptoms can include heavy and prolonged menstrual bleeding, severe pain, bloating and constipation or urinary complaints (2).
Treatment is reserved to women with substantial symptomatic fibroids. The most common treatment is hysterectomy, an invasive surgical procedure to remove the uterus. Hysterectomy is associated with surgical risks and complications, requires general anesthesia, a 3- to 4-day hospital stay, and results in a patient recovery time of 6 weeks or more. Up to a few years ago, hysterectomy was the gold standard for fibroids treatment: 199,000 hysterectomies for fibroids were performed in the United States in 1997 (2). Myomectomy is second to hysterectomy for fibroids treatment. This is a surgical procedure, less invasive than hysterectomy, that permits the removal of the fibroid only.
Today other less invasive techniques have been introduced, including UAE (4). UAE is a minimally invasive procedure; the goal of performing UAE is to produce infarction of the fibroids with consequent decrease in fibroid volume. UAE can treat more fibroids at the same time while preserving the uterus, it is done under spinal anesthesia and the patient can go home in 72 hours, however, it is a painful procedure because of the infarction of fibroids. As with any interventional procedure, there are a number of complications associated with UAE; the most common complications include fibroid expulsion (2.5%) and infections like endometritis (0.5%) (16).
MRI-guided focused ultrasound therapy for uterine fibroids is a noninvasive alternative to the traditional and most common treatments like hysterectomy and myomectomy, with low risk of complications and significant symptomatic improvement in most patients in a short time.
MRI-guided focused ultrasound ablation of uterine fibroids generates an area of coagulative necrosis within the fibroid, which results with contrast-enhanced MRI examination, in an area of non-enhancement of NPV. The necrotic area is absorbed with time; our study, confirming the results of previous research (4,17-20), has demonstrated a significant correlation between the quantity of necrotic tissue in the treated fibroid—therefore of NPV—and the reduction of its volume 6 months after treatment.
Moreover, greater shrinkage was found in patients with higher post-treatment NPV ratios.
The comparison of the NPV ratio distribution and the average fibroid shrinkage at 6 months between our data and the results of clinical trials in the world has shown analogous results in relation to studies published between 2009 and today. In our study the mean NPV immediately after treatment was 67%±20%, in agreement with LeBlang et al. (4) (55%±25%) and Dobrotwir et al. (17) (67%±25%), whereas the average volume reduction of fibroids 6 months after treatment was 37%±22% in our study, 31%±28% for Leblang et al. and 30%±11% for Desai et al. (18). Our results on post-treatment NPV and fibroid volume reduction at 6 months were substantially better than those reported in studies published between 2004 and 2007 (19-22). This can be due to the fact that in some of the first clinical trials patients were treated according to protocols constrained by operating restrictions (e.g., NPV <33%; treatment duration <120 min) imposed by Food and Drug Administration (FDA) guidelines (22).
Additional factors allowing for the greater treatment effectiveness in our study were the increased experience of the radiologist and a better selection of patients. Our treatment excluded non-hypointense fibroid on T2 weighted MRI, since previous studies have demonstrated that these fibroids do not respond as well to treatment (19).
Our study has also shown that volume reduction in fibroids, independently of its size, is accompanied by a significant reduction of patients’ symptoms at the 6-month follow-up, based on the UFS-QOL questionnaire. Furthermore we have observed that a greater non-perfused volume ratio after treatment correlates with greater shrinkage and symptoms improvement at the 6 months follow-up.
The mean SSS value at baseline was 53±23 and at 6 months follow-up is significantly decreased to 27±17 (P<0.001) with an important symptomatic reduction; the QOLS increased from 49±24 to 72±21 (P<0.001) with an improvement in the patients’ quality of life. These results are also favorably compared to the latest studies (17,18,23).
The low rate of side effects observed in our study and the quick return to routine activity are in contrast to the relatively high morbidity and need for variable times of hospitalization associated with the currently used surgical procedures for uterine fibroids or with UAE.
Limitations of our study include small population size and the great variability of treated fibroid size (range, 4.4-587 cm3) and, for extremely small fibroids, a greater margin of error in measuring their volumes in the post-processing phase. An additional limitation is certainly the lack of longer-term follow-up; however, the analysis of the preliminary results obtained 12 and 24 months after treatment is promising. So we believe that MRI-guided focused ultrasound therapy should be considered a safe and viable treatment option for symptomatic uterine fibroids in suitable patients and a valid therapeutic solution for women who want to preserve their uterus for future fertility.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giusi Irma Forte and Giorgio Russo) for the series “High intensity focused ultrasounds” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.10.03). The series “High intensity focused ultrasounds” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board and informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol 2005;48:312-24. [PubMed]
- Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007;87:725-36. [PubMed]
- Abdullah B, Subramaniam R, Omar S, et al. Magnetic resonance-guided focused ultrasound surgery (MRgFUS) treatment for uterine fibroids. Biomed Imaging Interv J 2010;6:e15 [PubMed]
- LeBlang SD, Hoctor K, Steinberg FL. Leiomyoma shrinkage after MRI-guided focused ultrasound treatment: report of 80 patients. AJR Am J Roentgenol 2010;194:274-80. [PubMed]
- Spies JB, Myers ER, Worthington-Kirsch R, et al. The FIBROID Registry: symptom and quality-of-life status 1 year after therapy. Obstet Gynecol 2005;106:1309-18. [PubMed]
- Okada A, Morita Y, Fukunishi H, et al. Non-invasive magnetic resonance-guided focused ultrasound treatment of uterine fibroids in a large Japanese population: impact of the learning curve on patient outcome. Ultrasound Obstet Gynecol 2009;34:579-83. [PubMed]
- Fennessy FM, Tempany CM. A review of magnetic resonance imaging-guided focused ultrasound surgery of uterine fibroids. Top Magn Reson Imaging 2006;17:173-9. [PubMed]
- Funaki K, Fukunishi H, Funaki T, et al. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol 2007;196:184.e1-6.
- Lénárd ZM, McDannold NJ, Fennessy FM, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery--imaging predictors of success. Radiology 2008;249:187-94. [PubMed]
- Smart OC, Hindley JT, Regan L, et al. Magnetic resonance guided focused ultrasound surgery of uterine fibroids--the tissue effects of GnRH agonist pre-treatment. Eur J Radiol 2006;59:163-7. [PubMed]
- Smart OC, Hindley JT, Regan L, et al. Gonadotrophin-releasing hormone and magnetic-resonance-guided ultrasound surgery for uterine leiomyomata. Obstet Gynecol 2006;108:49-54. [PubMed]
- Tempany CM, Stewart EA, McDannold N, et al. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology 2003;226:897-905. [PubMed]
- Zhang L, Chen WZ, Liu YJ, et al. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol 2010;73:396-403. [PubMed]
- Harding G, Coyne KS, Thompson CL, et al. The responsiveness of the uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL). Health Qual Life Outcomes 2008;6:99. [PubMed]
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290-300. [PubMed]
- Kitamura Y, Ascher SM, Cooper C, et al. Imaging manifestations of complications associated with uterine artery embolization. Radiographics 2005;25:S119-32. [PubMed]
- Dobrotwir A, Pun E. Clinical 24 month experience of the first MRgFUS unit for treatment of uterine fibroids in Australia. J Med Imaging Radiat Oncol 2012;56:409-16. [PubMed]
- Desai SB, Patil AA, Nikam R, et al. Magnetic Resonance-guided Focused Ultrasound Treatment for Uterine Fibroids: First Study in Indian Women. J Clin Imaging Sci 2012;2:74. [PubMed]
- Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol 2004;183:1713-9. [PubMed]
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol 2007;30:771-7. [PubMed]
- Stewart EA, Gostout B, Rabinovici J, et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol 2007;110:279-87. [PubMed]
- Fennessy FM, Tempany CM, McDannold NJ, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery--results of different treatment protocols. Radiology 2007;243:885-93. [PubMed]
- Kim HS, Baik JH, Pham LD, et al. MR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: long-term outcomes. Acad Radiol 2011;18:970-6. VV. [PubMed]


