
Incidence, treatment, and survival analysis in mediastinal malignant teratoma population
Introduction
Mediastinal tumors are frequently seen in daily clinical practice. In contrast, mediastinal malignant teratoma is very rare. Mediastinal malignant teratoma can contain ectodermal, mesodermal, and endodermal derivatives from three germ layers, which can often be seen on imaging by presence of fat, soft tissue and calcium.
There is little research on mediastinal malignant teratoma (1). The incidence of malignant teratoma is highest in young adults aged 18 to 39. Factors including later age at diagnosis, advanced stage, and high-grade histology are conventionally thought to confer a worse prognosis (2). Treatments of mediastinal malignant teratoma have not been well studied. Current treatments mainly include both surgery and chemotherapy (3,4). It is well acknowledged that surgery can bring survival benefits. However, the role of chemotherapy in mediastinal malignant teratoma is still vague. Little research has paid attention to the role of radiotherapy in mediastinal malignant teratoma. In addition, there is also a lack of population-based analysis on mediastinal malignant teratoma due to its rarity.
The aim of this study is to evaluate the incidence, survival and treatment of mediastinal malignant teratoma via the Surveillance, Epidemiology and End Results (SEER) registry. SEER database is a robust platform in analyzing information of rare cancers. We analyzed clinicopathological characteristics of malignant teratoma population. We also compared different prognosis of treatments including surgery, chemotherapy and radiotherapy. Therefore, this study can improve the understanding on mediastinal malignant teratoma.
Methods
Patient and tumor characteristics
From SEER 18 database, we extracted information of patients with malignant teratoma between 1975 and 2016, and analyzed incidence, frequency, and survival data. Because of hidden patient identifiers, there is no need for this study to get the approval of Institutional Review Board.
We used behavior code 3 to identify the malignant tumors. After the filtration, we selected cases characterized by the following site-specific codes of mediastinum: C38.1-Anterior mediastinum, C38.2-Posterior mediastinum, C38.3-Mediastinum, NOS, C38.8-Overlapping lesion of heart, mediastinum and pleura. These codes did not include thymus primary site (C37.9), trachea primary site (C33.9), heart-only primary site (C38.0), or esophagus primary site (C15.0–15.9). Based on International Classification of Disease for Oncology, 3rd Edition (ICD-O-3), we used histology/behavior codes 9080–9084 and 9102 representing different teratoma subtypes to select patients with IT (excluded mixed germ cell tumors). Cases were excluded from the study cohort, if tumors were not first appearing, not malignant, and without histological/microscopic confirmation.
We conducted classification and statistical analysis, based on clinicopathological characteristics, including gender, ethnicity, marital status, age, tumor location and grade. We applied tumor-node-metastasis (TNM) staging system, American Joint Committee on Cancer (AJCC) staging system, SEER histological staging system and intervention types for describing tumor features. Right-censored data of the overall survival (OS) and cancer-specific survival (CSS) analysis contained cases that survived to the deadline, lost follow-up or died of other reasons.
Incidence and survival
Rates were reported per 100,000 persons, and age of the patients was adjusted to the 2000 US Standard Population (19 age groups, census P25-1130) standard. Annual percentage change (APC) was demonstrated in incidence using 1-year endpoints to analyze survival rate. OS and CSS were used to construct Kaplan-Meier model and reflect prognosis.
Statistical analysis
After extracting data of frequency, incidence, and survival from the SEER 18 database, we conducted statistical analysis by using SPSS software (version 20.0, SPSS Inc., Chicago, IL, USA) and SEER*Stat 8.3.5 software (National Cancer Institute, Bethesda, Maryland). Data of incidence were analyzed by weighted least squares to generate APC, based on 1-year endpoints in SEER*Stat 8.3.5 software. The student’s t-test and Chi-square test were respectively used for dealing with continuous and categorical variables. P values were two-tailed. P<0.05 were defined to be statistically significant.
Results
Patient characteristics
In the SEER 18 database, a total of 5,550 patients were identified with mediastinal malignant teratoma (n=133) and other malignant teratomas (n=5,417) during 1975 to 2016 (Figure 1). All demographic characteristics were shown in Table 1. Noticeably, patients with mediastinal malignant teratoma (median: 11 cm; mean: 11.3 cm) had larger tumor size, compared with patients with other malignant teratomas (median: 5.5 cm; mean: 8 cm) (P<0.001). Mediastinal malignant teratoma (81.2%) preferred male patients (P=0.001). Moreover, patients with mediastinal malignant teratoma had less proportion of receiving surgery (75.9%) but larger proportion of receiving chemotherapy (69.2%) and radiotherapy (19.7), compared with those with other malignant teratomas (all P<0.001). No significant difference existed in patient age or ethnicity.
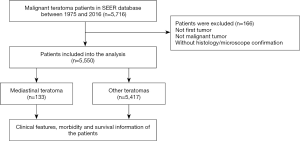
Table 1
| Factor, n (%) | Subcategory | Mediastinal teratoma (n=133) | Other teratoma (n=5,417) | P value |
|---|---|---|---|---|
| Year (%) | 1975–1986 | 41 (30.8) | 1,256 (23.2) | 0.060 |
| 1987–1996 | 23 (17.3) | 1,146 (21.2) | ||
| 1997–2006 | 29 (21.8) | 1,590 (29.4) | ||
| 2007–2016 | 40 (30.1) | 1,425 (26.3) | ||
| Age (years) | Median | 19.0 | 24.0 | 0.352 |
| Mean | 19.9 | 24.56 | ||
| Range | 0–55 | 0–83 | ||
| Size (cm) | Median | 11.0 | 5.5 | <0.001 |
| Mean | 11.3 | 8.0 | ||
| Range | 0.4–25 | 0.1–98.9 | ||
| Sex (%) | Male | 108 (81.2) | 3,647 (67.3) | 0.001 |
| Female | 25 (18.8) | 1,770 (32.7) | ||
| Race/ethnicity (%) | White | 107 (80.5) | 4,552 (84.0) | 0.343 |
| Black | 9 (6.8) | 373 (6.9) | ||
| Other | 17 (12.8) | 492 (9.1) | ||
| Surgery (%) | Yes | 100 (75.2) | 5,238 (96.7) | <0.001 |
| No | 33 (24.8) | 155 (2.9) | ||
| Chemotherapy (%) | Yes | 92 (69.2) | 2,152 (39.7) | <0.001 |
| No | 41 (30.8) | 3,265 (60.3) | ||
| Radiotherapy (%) | Yes | 26 (19.5) | 198 (3.7) | <0.001 |
| No | 107 (80.5) | 5,202 (96.0) | ||
| Incidence (2016) | – | 0.004 | 0.147 | – |
| Annual percentage change (2000–2016) | – | −1.727 (P=0.528) | −2.754 (P<0.001) | – |
Tumor characteristics
In Table 2, we summarized TNM, SEER, and AJCC stages of mediastinal malignant teratoma. In detail, for T stage, T2 was the most identified (47.6%), followed by stage of T1 (23.8%). For N stage, N0 and N1 were 66.7% and 9.5%, respectively. For M stage, metastasis (M1) only appeared in 14.3% of cases, while 85.7% of cases were without distant metastasis. Similarly, SEER stages revealed that 27.7% of tumors were with distant metastasis. In AJCC staging system, the largest proportion was belonged to stage I (52.4%), followed by stage IV (14.3%). As for tumor differentiation, grades of I, II, III, and IV were 2.3%, 3.8%, 5.3% and 3.8%, respectively.
Table 2
| Parameters | Number | Percent (%) |
|---|---|---|
| TNM | ||
| T stage | ||
| T1 | 5 | 23.8 |
| T2 | 10 | 47.6 |
| T3 | 0 | 0 |
| T4 | 0 | 0 |
| Unknown | 6 | 28.6 |
| N stage | ||
| N0 | 14 | 66.7 |
| N1 | 2 | 9.5 |
| N2 | 0 | 0 |
| N3 | 0 | 0 |
| Unknown | 5 | 23.8 |
| M stage | ||
| M0 | 18 | 85.7 |
| M1 | 3 | 14.3 |
| SEER stage | 83 | |
| Localized | 28 | 33.7 |
| Regional | 26 | 31.3 |
| Distant | 23 | 27.7 |
| Unstaged | 6 | 7.2 |
| AJCC stage | ||
| I | 11 | 52.4 |
| II | 1 | 4.8 |
| III | 2 | 9.5 |
| IV | 3 | 14.3 |
| Unknown | 4 | 19.0 |
| Grade | ||
| I | 3 | 2.3 |
| II | 5 | 3.8 |
| III | 7 | 5.3 |
| IV | 5 | 3.8 |
| Unknown | 113 | 85.0 |
TNM, tumor-node-metastasis; SEER, surveillance, epidemiology and end results; AJCC, American Joint Committee on Cancer.
Incidence analysis
The incidence of mediastinal malignant teratoma was verified to be 0.004 per 100,000 persons in 2016, after age adjustment to the 2000 US Standard Population (19 age groups, census P25-1130) standard (Table 1). Between 2000 and 2016, mediastinal malignant teratoma exhibited steady changing rate (APC: −1.727; P=0.528), while other malignant teratomas had a decreasing tendency (APC: −2.754; P<0.001) (Table 1, Figure 2).

Treatments and survival analysis
In Table 3, we summarized the profile of treatments among patients with mediastinal malignant teratoma. Among all treatments, only surgery showed protection for patients with mediastinal malignant teratoma [univariate analysis: hazard ratio (HR) =0.34 and P<0.001; multivariate analysis: HR =0.44 and P=0.006]. In contrast, radiotherapy exhibited detrimental effect on OS (univariate analysis: HR =1.83 and P=0.016). In addition, chemotherapy had no impact on OS (P=0.12). Furthermore, tumor grades did not influence the OS of patients with mediastinal malignant teratoma (P=0.173). Likewise, AJCC stages analysis of mediastinal malignant teratoma did not show difference between stage I/II and stage III/IV (P=0.105). However, under SEER staging system, distant/unstaged tumor seemed to harsh the OS (univariate analysis: HR =2.74 and P<0.001; multivariate analysis: HR =2.7 and P=0.001), compared with localized/regional tumor. Moreover, aging (univariate analysis: HR =1.02 and P<0.001; multivariate analysis: HR =2.67 and P=0.001) and male (univariate analysis: HR =4.89 and P=0.001; multivariate analysis: HR =5.39 and P=0.006) were found to be unfavorable prognostic factors in OS of patients with mediastinal malignant teratomas.
Table 3
| Parameters | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | ||
| Age | <0.001 | 1.02 (1.01–1.04) | 0.001 | 2.67 (1.51–4.74) | |
| Year | |||||
| 1975–1995 | Reference | – | – | ||
| 1996–2016 | 0.058 | 0.65 (0.42–1.02) | – | – | |
| Gender | |||||
| Female | Reference | Reference | |||
| Male | 0.001 | 4.89 (1.98–12.10) | 0.006 | 5.39 (1.64–17.72) | |
| Surgery | |||||
| No | Reference | Reference | |||
| Yes | <0.001 | 0.34 (0.21–0.54) | 0.006 | 0.44 (0.25–0.79) | |
| Chemotherapy | |||||
| No | Reference | – | – | ||
| Yes | 0.12 | 1.54 (0.89–2.67) | – | – | |
| Radiotherapy | |||||
| No | Reference | – | – | ||
| Yes | 0.016 | 1.83 (1.12–3.00) | – | – | |
| Grade | |||||
| I/II | Reference | – | – | ||
| III/IV | 0.173 | 2.44 (0.68–8.80) | – | – | |
| SEER historic stage | |||||
| Localized/regional | Reference | Reference | |||
| Distant/unstaged | <0.001 | 2.74 (1.54–4.58) | 0.001 | 2.70 (1.53–4.78) | |
| AJCC stage | |||||
| I/II | Reference | – | – | ||
| III/IV | 0.105 | 6.51 (0.68–62.71) | – | – | |
HR, hazard ratio; CI, confidence interval; SEER, surveillance, epidemiology and end results; AJCC, American Joint Committee on Cancer.
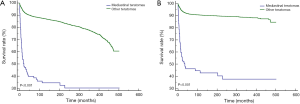
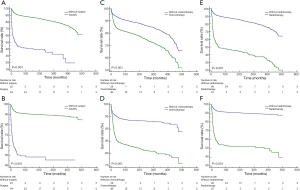
For both OS (P<0.001) and CSS (P<0.001), mediastinal malignant teratomas demonstrated significantly worse prognosis than other malignant teratomas (Figure 3). Subsequently, we conducted survival analysis to evaluate the effect of different treatment strategies on mediastinal malignant teratomas. As a result, surgery was proved to benefit both OS (P<0.001) and CSS (P<0.001) of patients with mediastinal malignant teratomas (Figure 4A,B). However, chemotherapy and radiotherapy could negatively influence both OS and CSS of patients with mediastinal malignant teratomas (all P<0.001) (Figure 4C,D,E,F). Since the most patients with mediastinal malignant teratoma were diagnosed at early stage (T1/T2, N0 and M0 stages) (Table 2), we also conducted survival analysis (OS, CSS) among such early-staged subpopulation to minimize the selection bias. As expected, surgery still exhibited protective effect to both OS (P<0.001) and CSS (P<0.001), although chemotherapy and radiotherapy did not influence early-staged patient survival (Figure S1).
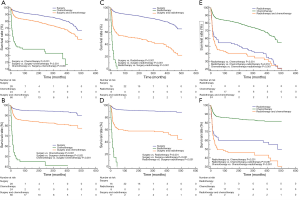
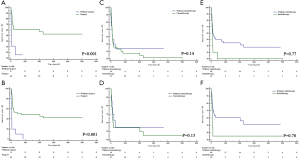
Moreover, we compared effects of different single or combinational therapies on mediastinal malignant teratomas. In detail, as we expected, surgery can bring better OS and CSS for patients with mediastinal malignant teratomas, compared with chemotherapy and radiotherapy (Figure 5A,B,C,D). Compared with radiotherapy, chemotherapy showed more advantages in OS and CSS of patients with mediastinal malignant teratomas (Figure 5E,F). As for combinational therapies, surgery + chemotherapy performed better than single use of chemotherapy but worse than surgery alone in-patient survival (Figure 5A,B). Likewise, surgery + radiotherapy also performed better than single use of chemotherapy but worse than surgery alone in-patient survival (Figure 5C,D). For OS and CSS of patient with mediastinal malignant teratoma radiotherapy + chemotherapy exhibited less survival benefit compared with chemotherapy alone, and no difference compared with chemotherapy alone (Figure 5E,F).
Discussion
Teratomas are germ cell tumors containing tissues from all three germinal layers of the endoderm, mesoderm, and ectoderm. The most common localization is the ovary or the testis (5). Based on their histological characteristics, teratomas are divided into three categories, including mature (benign) teratoma, immature (malignant) teratoma, and teratoma with additional malignant components (6). The mechanisms of teratomas have been illustrated at different levels. Previous study found uneven number of chromosomes could cause interference of tumor proliferation and differentiation, which may be related with the formation of poorly differentiated teratomas (7). Other studies paid attention to the role of Cyclin family, like Cyclin D1, and its related molecules, like CDKN2B and CDK1, during the development of teratomas (8-10). Other molecules, like Geminin, could also get involved in the formation of teratoma (11).
Malignancy is observed in 27% of teratomas and mediastinum is the most common extra-gonadal site of malignant teratomas (5,12,13). Malignant teratomas were usually defined by the presence of immature tissues rather than invasiveness or metastasis. In this study, both mediastinal malignant teratoma and other teratomas shared a decreasing tendency, although only the incidence of other teratomas was statistically significant (Figure 2). On the one hand, such gradual improvement may be attributed to the reduced smoking rate among the US population, which could also be observed in other types of cancer as well. On the other hand, the seemingly steady incidence rate of mediastinal malignant teratoma may be merely due to the disease rarity. Moreover, previous study reported the incidence of malignant teratoma is highest in young adults aged 18 to 39, which is similar to our finding from the study cohort (Table 1) (2). This study also demonstrated that later age at diagnosis and male can serve as poor prognostic factors for mediastinal malignant teratoma. Meanwhile, despite malignancy prevalence is conventionally thought not to be correlated to the size of the tumor, we did find the size of mediastinal malignant teratoma is significantly larger than that of other malignant teratomas (Table 1). We reasoned that such difference in tumor size may be associated with the unique anatomical sites bearing tumors and/or the other factors including sex predilection, which may participate in the genetic or epigenetic changes of tumors. Detailed studies are still warranted in the future. Besides, although teratoma malignancy is associated with tumor differentiation status and traditional idea supports that high-grade histology confer a worse prognosis, we did not find evidence supporting tumor grade as a prognostic factor (Table 3). Besides, while both previous reports and this study support that metastasis is harmful (Table 3), only a small proportion of patients have tumor metastasis (Table 2) (1). What determines the metastasis of mediastinal malignant teratoma remains largely unknown.
Compared with other malignant teratomas, mediastinal malignant teratomas have worse survival (Figure 3). Such disadvantage may be partly attributed to patient untypical symptoms including chest pain, fever, cough, sputum, and hemoptysis. It should be noticed that some tumors may contain digestive tract tissue that can produce proteolytic enzymes, which makes them prone to rupture and lead to severe complications (14). However, in our study cohort, most patients with mediastinal malignant teratoma were diagnosed at early stage (Table 2). Based on TNM staging system, we found most patients with mediastinal malignant teratoma were diagnosed at T1/T2, N0 and M0 stages (Table 2). Several examinations can help early diagnosis of mediastinal malignant teratomas. Serologic test, like alpha-fetoprotein and β-human chorionic gonadotropin, is helpful in diagnosis (15). Recent studies also suggested miR-371 and miR-302 as plasma biomarkers (16,17). Besides, imaging is essential for early diagnosis (18). Both chest X-ray and computed tomography can help diagnose mediastinal teratoma (19,20). Noticeably, after receiving imaging results, it is important for clinicians to make differential diagnosis from other chest diseases including aneurysms of aorta and tuberculosis (21,22). In addition, CT- or ultrasound-guided biopsy can help yield a precise diagnosis of mediastinal malignant teratomas (23).
To date, surgery is still the prior option in treating mediastinal malignant teratoma (22). As we expected, this study revealed that surgery is a significant protective factor for either early-staged or all-staged mediastinal malignant teratoma, which agrees with previous report (Table 3, Figures 4,S1) (24). Conventionally, complete surgical resection is common choice for such tumor, although this is sometimes difficult and requires careful attention to mediastinal structures like the phrenic nerve, vagus nerve, or hilar structures (3,4,25). With improving medical technologies and surgical experience, minimally invasive surgical techniques, such as video-assisted thoracoscopic surgery or robotic surgery, are starting to be recommended when the mass is small, while invasive techniques like median sternotomy are still applied for the large mass (26). However, the most recent studies also supported video-assisted thoracic surgery in certain patients with large masses (27).
This study also explored the effect of other treatments, including chemotherapy and radiotherapy. Chemotherapy is common treatment for mediastinal malignant teratoma. However, in this study, chemotherapy is detrimental for both OS and CSS (Figure 4C,D). Moreover, adjuvant therapy (surgery + chemotherapy) did not show any advantage but even harmed patient prognosis (Figure 5A,B). Such finding may break conventional idea. Clinically, a combined approach of surgery and chemotherapy has often been recommended for mediastinal malignant teratoma. Yet, mediastinal malignant teratoma can be chemotherapy-resistant and protocols like cisplatin-based chemotherapy may not be effective (28). Pluripotency of the tumor component allow histological malignant transformations inside the tumor. Although chemotherapy could eradicate components like yolk-sac, chemo-resistance may happen because of somatic-type malignancy, the non-germ cell tumor component, inside the tumor (28). There is little research of the effect of radiotherapy on mediastinal malignant teratomas. Noticeably, this study demonstrated that it is even more harmful than chemotherapy with respect to patient survival (Figure 5E,F). In addition, emerging evidence recently indicates that immunological therapy, such as monoclonal antibody, could be potentially used to kill human embryonic stem cells in vivo, and thus prevent or delay the formation of teratoma (29).
Admittedly, our research still has limitations, although population-based data were used. First, we cannot directly observe pathological slices and immunohistochemistry results in SEER database. Second, details including surgery types, surgical skills, surgical experience, staging methods, drug use, radiotherapy protocols, and comorbidities were missed that hindered our further analysis. In turn, specific diagnostic and therapeutic methods could evolve and therefore introduce temporal heterogeneity during the analyzed period (1975–2016) Third, biases can be introduced because of data input under different circumstances (e.g., tumor stage and grade information are missing in a considerable proportion of patients) or by different recorders. Even though, using the SEER database can yield great benefits. Such standardized database can help eliminate biases caused by geographical and institutional difference during analysis. Moreover, the relatively large number of patients can help researchers to make confident conclusions in analyzing rare malignancies such as mediastinal malignant teratoma.
In summary, this study shows that the incidence of mediastinal malignant teratoma is very low. Compared with other malignant teratomas, mediastinal malignant teratoma had worse prognosis. Surgery is still the mainstay of the treatment for mediastinal malignant teratoma. Combinational strategy, like surgery + chemotherapy/radiotherapy, cannot improve but harm patient prognosis.
Conclusions
Our population-based evidence showed that mediastinal malignant teratoma, a rare cancer, had worse prognosis compared with other malignant teratomas. Compared with chemotherapy and radiotherapy, surgery could yield more survival benefits for either early-staged or all-staged patients with mediastinal malignant teratoma. Adjuvant use of chemotherapy/radiotherapy to surgery cannot improve but potentially harm patient prognosis
Acknowledgments
We would like to extend our appreciations to the SEER database.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.19). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mustafa OM, Mohammed SF, Aljubran A, et al. Immature mediastinal teratoma with unusual histopathology: A case report of multi-lineage, somatic-type malignant transformation and a review of the literature. Medicine (Baltimore) 2016;95:e3378. [Crossref] [PubMed]
- Jorge S, Jones NL, Chen L, et al. Characteristics, treatment and outcomes of women with immature ovarian teratoma, 1998-2012. Gynecol Oncol 2016;142:261-6. [Crossref] [PubMed]
- Kim D, Kim SW, Hong JM. Rapid growing huge teratoma: complete surgical resection. J Thorac Dis 2014;6:E217-9. [PubMed]
- Zhao H, Zhu D, Zhou Q. Complete resection of a giant mediastinal teratoma occupying the entire right hemithorax in a 14-year-old boy. BMC Surg 2014;14:56. [Crossref] [PubMed]
- Guibert N, Attias D, Pontier S, et al. Mediastinal teratoma and trichoptysis. Ann Thorac Surg 2011;92:351-3. [Crossref] [PubMed]
- Moran CA, Suster S. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer 1997;80:681-90. [Crossref] [PubMed]
- Storchova Z. Too much to differentiate: aneuploidy promotes proliferation and teratoma formation in embryonic stem cells. EMBO J 2016;35:2265-7. [Crossref] [PubMed]
- Zheng Z, Li C, Ha P, et al. CDKN2B upregulation prevents teratoma formation in multipotent fibromodulin-reprogrammed cells. J Clin Invest 2019;129:3236-51. [Crossref] [PubMed]
- Huskey NE, Guo T, Evason KJ, et al. CDK1 inhibition targets the p53-NOXA-MCL1 axis, selectively kills embryonic stem cells, and prevents teratoma formation. Stem Cell Reports 2015;4:374-89. [Crossref] [PubMed]
- Lanza DG, Dawson EP, Rao P, et al. Misexpression of cyclin D1 in embryonic germ cells promotes testicular teratoma initiation. Cell Cycle 2016;15:919-30. [Crossref] [PubMed]
- Adler-Wailes DC, Kramer JA, DePamphilis ML. Geminin Is Essential for Pluripotent Cell Viability During Teratoma Formation, but Not for Differentiated Cell Viability During Teratoma Expansion. Stem Cells Dev 2017;26:285-302. [Crossref] [PubMed]
- Rusner C, Trabert B, Katalinic A, et al. Incidence patterns and trends of malignant gonadal and extragonadal germ cell tumors in Germany, 1998-2008. Cancer Epidemiol 2013;37:370-3. [Crossref] [PubMed]
- Stang A, Trabert B, Wentzensen N, et al. Gonadal and extragonadal germ cell tumours in the United States, 1973-2007. Int J Androl 2012;35:616-25. [Crossref] [PubMed]
- Asano S, Hoshikawa Y, Yamane Y, et al. An intrapulmonary teratoma associated with bronchiectasia containing various kinds of primordium: a case report and review of the literature. Virchows Arch 2000;436:384-8. [Crossref] [PubMed]
- Pendlebury A, Rischin D, Ireland-Jenkin K, et al. Ovarian Growing Teratoma Syndrome With Spuriously Elevated alpha-Fetoprotein. J Clin Oncol 2015;33:e99-100. [Crossref] [PubMed]
- Li HL, Wei JF, Fan LY, et al. miR-302 regulates pluripotency, teratoma formation and differentiation in stem cells via an AKT1/OCT4-dependent manner. Cell Death Dis 2016;7:e2078. [Crossref] [PubMed]
- Salvatori DCF, Dorssers LCJ, Gillis AJM, et al. The MicroRNA-371 Family as Plasma Biomarkers for Monitoring Undifferentiated and Potentially Malignant Human Pluripotent Stem Cells in Teratoma Assays. Stem Cell Reports 2018;11:1493-505. [Crossref] [PubMed]
- Xiao-Dong L, Li Z, Xiu-Mei D, et al. Identification of a giant mediastinal teratoma by echocardiography: A case report. J Clin Ultrasound 2019;47:380-3. [Crossref] [PubMed]
- Jones LM, Bradshaw DA. Images in clinical medicine. Benign mediastinal teratoma. N Engl J Med 2008;359:841. [Crossref] [PubMed]
- Li W, Zhang L, Zhang R. Increased 99mTc-MDP Activity in a Partially Calcified Malignant Mediastinal Teratoma. Clin Nucl Med 2016;41:161-3. [Crossref] [PubMed]
- Liu J, Tian B, Zeng Q, et al. Mediastinal teratoma presenting with hemoptysis and pleuritis misdiagnosed as tuberculosis (empyema). BMC Pediatr 2018;18:382. [Crossref] [PubMed]
- Bhat V, Belaval V, Binoy C, et al. Anterior mediastinal teratoma presenting with pseudo-aneurysms of aorta. Eur Heart J Cardiovasc Imaging 2014;15:227. [Crossref] [PubMed]
- Resnick EL, Talmadge JM, Winn SS. Mediastinal teratoma diagnosed via ultrasound-guided biopsy. Ultrasound Q 2013;29:245-6. [Crossref] [PubMed]
- Schneider DT, Calaminus G, Reinhard H, et al. Primary mediastinal germ cell tumors in children and adolescents: results of the German cooperative protocols MAKEI 83/86, 89, and 96. J Clin Oncol 2000;18:832-9. [Crossref] [PubMed]
- Yendamuri S. Resection of a Giant Mediastinal Teratoma. Ann Thorac Surg 2016;102:e401-2. [Crossref] [PubMed]
- Willems E, Martens S, Beelen R. Robotically enhanced mediastinal teratoma resection: a case report and review of the literature. Acta Chir Belg 2016;116:309-12. [Crossref] [PubMed]
- Hwang SK, Park SI, Kim YH, et al. Clinical results of surgical resection of mediastinal teratoma: efficacy of video-assisted thoracic surgery. Surg Endosc 2016;30:4065-8. [Crossref] [PubMed]
- Ulbright TM, Loehrer PJ, Roth LM, et al. The development of non-germ cell malignancies within germ cell tumors. A clinicopathologic study of 11 cases. Cancer 1984;54:1824-33. [Crossref] [PubMed]
- Tan HL, Tan BZ, Goh WXT, et al. In vivo surveillance and elimination of teratoma-forming human embryonic stem cells with monoclonal antibody 2448 targeting annexin A2. Biotechnol Bioeng 2019;116:2996-3005. [Crossref] [PubMed]


