ET-1 promotes the growth and metastasis of esophageal squamous cell carcinoma via activating PI3K/Akt pathway
Introduction
Esophageal squamous cell carcinoma (ESCC) is a common malignant tumor of the digestive tract and an important health-related problem in the world (1). China is one of the countries with the highest incidence and mortality of esophageal cancer, with ESCC as the main subtype (2). Although progress in the diagnosis and treatment of ESCC has been made in recent years, the prognosis is still poor and the 5-year survival rate is less than 30%, mainly because many patients are diagnosed at an advanced stage. ESCC in the early stage has no obvious clinical symptoms and a lack of effective and non-invasive methods for the early diagnosis (3). Therefore, it is urgent to better understand the molecular mechanism and identify biomarkers for the diagnosis, prognosis and treatment of ESCC.
Endothelin-1 (ET-1), as a member of endothelin families including three 21-amino acid peptides, is the most potent vasoconstrictor agent ever identified and participated in the progression of various diseases including pulmonary artery hypertension (4), cardiovascular disease (5), atherosclerosis (6) and osteoarthritis (7). Recently, several studies have proved the aberrant expression of ET-1 in multiple cancers such as ovarian cancer (8), prostate cancer (9), breast cancer (10) and colon cancer (11). Moreover, ET-1 plays an important role in the development and progression of various tumors through mediating cell proliferation, migration and invasion, for example, knockdown of ET-1 significantly inhibited lung cancer cell proliferation and invasion (12). Said et al. showed that silencing of ET-1 decreased tumor metastasis of bladder cancer (13) and osteosarcoma (14). However, the value and mechanism of ET-1 in the progression of ESCC remains poorly understood.
Increasing evidence confirmed that the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signaling pathway, as a regulatory factor of tumor progression, was activated in various cancers (15,16). For example, inhibition of the PI3K/Akt signaling pathway suppressed cell proliferation, invasion and migration of colorectal cancer (17), lung cancer (18), oral squamous cell carcinoma (19), hepatocellular carcinoma (20) and ESCC (21), etc. Moreover, several tumor-related genes (TRE), as the upstream regulator of the PI3K/Akt signaling pathway, were involved in tumor progression and development via regulating cancer cell malignant biological behavior, for instance, upregulation of gene of phosphate and tension homology deleted on Chromosome 10 (PTEN) inhibited tumor progression and enhanced the sensitivity of cancer cell to chemotherapy through blocking the PI3K/Akt signaling pathway (22,23). Wang et al. showed that human ether a-go-go-related gene 1 (HERG1) promoted ESCC cell proliferation and metastasis through activating the PI3K/Akt signaling pathway (24). Wei et al. found that Yes-associated protein (YAP) suppressed the progression of ovarian cancer by inactivating the PI3K/Akt signaling pathway (25). Interestingly, previous studies have proved that the function of ET-1 in the progression of various diseases by regulating the PI3K/Akt signaling pathway, for example, inhibition of ET-1 alleviated the pathophysiology of amyotrophic lateral sclerosis by blocking the PI3K/Akt signaling pathway (26). Horowitz et al. reported that overexpression of ET-1 promoted myofibroblast resistance to apoptosis through inhibiting the PI3K/Akt signaling pathway (27). However, the effect of ET-1 on tumor growth and metastasis of ESCC via regulating the PI3K/Akt signaling pathway remains elusive.
In the present study, the expression of ET-1 was determined in ESCC tissues and cell lines. Moreover, we further to examine the role of ET-1 in the proliferation, cell cycle, migration, and invasion of ESCC cells. Mechanistically, to explore whether ET-1 regulated the tumor growth and metastasis of ESCC through PI3K/Akt pathway. Collectively, our studies may provide an important indicator of prognosis and guidance for ESCC clinical treatment.
Methods
Clinical samples
The ESCC tissues and their paired normal tissues were collected from the patients (n=45) from 2012 to 2017 at The First Affiliated Hospital of Nanchang University, none of the patients received chemotherapy or radiotherapy prior to surgery. The clinical specimens were immediately frozen at –80 °C refrigerator after resection and prepared for further experiments. Besides, all the patients have signed the informed consent and the approval was also obtained from the Ethics Committee of The First Affiliated Hospital of Nanchang University. All the involved clinical experiments were conducted in accordance with the principle of the “Declaration of Helsinki”.
Cell culture
The human ESCC cell lines (TE-1, ECA109, KYSE150, CaES-17, and EC9706), human normal esophageal epithelial cell line Het-1A and HEK-293 cells were purchased from Shanghai Institutes for Biological Sciences of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, USA) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, USA) in the incubator with a humidified atmosphere containing 5% CO2 at 37 °C.
Transfection
The ET-1 siRNA and scrambled negative control RNA were designed and constructed by Shanghai GenePharma Co., Ltd (Shanghai, China). All the above vectors were transfected into the ECA109 cells using the Lipofectamine 3000 kit purchased from Invitrogen (Thermo Fisher Scientific, USA) according to the manufacturer’s instruction.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA of tissues and cell lines were extracted using the Trizol reagent purchased from Life Technologies (USA). After that, the TaqMan Reverse Transcription Reagents (Applied Biosystems, USA) were employed to reversely transcribed the total RNA to complementary DNA (cDNA). The SYBR Green PCR Master Mix (Applied Biosystems, USA) was next used to quantify the expression levels of targeted genes at transcriptional levels. The relative mRNA levels were normalized to β-actin. The sequences of the involved primers were listed in Table 1.
Table 1
| Gene | Forward | Reverse |
|---|---|---|
| ET-1 | 5'-GCCTGTCTGAAGCCATAG-3' | 5'-GCTGAGAGGTCCATTGTC-3' |
| β-actin | 5'-CTCCATCCTGGCCTCGCTGT-3' | 5'-GCTGCTACCTTCACCGTTCC-3' |
Western blot
The polyvinylidene fluoride (PVDF) membranes were incubated overnight at 4 °C with the primary antibodies ET-1 (1:1,000, #ab117529, Abcam, USA), p-PI3K (1:1,000, #17366, Cell Signaling Technology, USA), PI3K (1:1,000, #4249, Cell Signaling Technology, USA), p-Akt473 (1:2,000, #4060, Cell Signaling Technology, USA), p-Akt308 (1:1,000, #13038, Cell Signaling Technology, USA), Akt (1:1,000, #4685, Cell Signaling Technology, USA) and β-actin (1:1,000, #ab5694, Abcam, USA). The horseradish peroxidase-coupled secondary antibody (anti-rabbit immunoglobulin (Ig)G (H+L); 1:3,000, #14708, Cell Signaling Technology, USA) was incubated with the membranes for 1 h at room temperature. The electrochemiluminescence (ECL) Western blot detection kit (Bio-Rad, USA) was employed to detect the optical density (OD) of the protein bands to evaluate the expression levels of the protein proteins.
Cell counting Kit-8 (CCK-8) assay
The ECA109 cells were cultured under the standard conditions and transfected with different vectors until the cell confluency reached about 70–80%. After that, the cells were collected and seeded in the 96-well plates at the density of 5,000 cells per well. After that, the CCK-8 kit (AbMole, USA) was employed to determine cell proliferation according to the manufacturer’s protocol. Briefly, the CCK-8 solution (10 µL per well) was added into the wells and incubated with the cells for 2 h. The OD values were then detected in the wavelength of 450 nm to evaluate cell proliferative ability.
Flow cytometry analysis
Collected the samples mentioned above, washed with PBS, centrifuged at 800 ×g for 6 min, suspended in ice-cold 70% ethanol/phosphate buffered saline (PBS), centrifuged at 800 ×g for another 6 min, and suspended with PBS. The cell cycle of ECA109 cells was verified with the Cell Cycle kit (Multiscience Biotech, China) by cytometry. The cytometry was executed with Beckman CytoFLEX FCM.
Transwell migration and invasion assay
The ECA109 cells were transfected with different vectors and collected until the cell confluency reached about 70−80%. Paced the 2×104 cells on the surface of the Transwell upper chamber. For invasion assay, Matrigel (BD Biosciences, USA) was coated the upper surface of polycarbonate filters. After 24 h, the invaded and migrated cells were fixed with 4% paraformaldehyde and rinsed three times with PBS, then stained with 0.1% crystal violet for 10 min and rinsed three times with PBS. Random selection 5 fields of vision for cell count, observation and photography.
Animal model
All animal experiments were performed in accordance with the Laboratory Animal Care guidelines of Animal Ethics Committee of The First Affiliated Hospital of Nanchang University. ECA109 cells were pre-transfected with ET-1 siRNA for 24 h. Afterward, 200 µL cells (1×107) were injected subcutaneously into the flanks of 6-week-old female nude mice (8 mice per group) and allowed to form tumors. Tumors' length and width were measured every three days. After 4 weeks of incubation, the tumor tissues were collected for further experiments, such as the immunohistochemistry staining was performed to detect the expression of Ki-67 (1:1,500, Abcam, USA) according to the previous studies (28).
Statistical analysis
The data in this study are presented as mean ± standard deviation (SD) of 3 or 8 individual experiments and analyzed by a one-way analysis of variance (ANOVA) test, and the difference between the two groups was determined using the Student t-test, which was considered statistically significantly P<0.05 or P<0.01. Survival data were estimated using the Kaplan-Meier survival curves and analyzed using the log-rank test.
Results
ET-1 was overexpressed in ESCC and associated with poor outcome
Previous studies confirmed that ET-1 plays an important role involved in the progression of multiple tumors (29), we examined the mRNA expression level of ET-1 in ESCC tissues and cell lines. As expected, ET-1 was significantly overexpressed in ESCC tissues relative to the adjacent tissues (P<0.001, Figure 1A). Immunohistochemical staining showed that ET-1 was expressed significantly higher in ESCC tumors than in the adjacent tissues (Figure 1B). Meanwhile, high expression of ET-1 was positively correlated with tumor size (P<0.05), tumor stage (P<0.01), and tumor metastasis (P<0.05) by comparing clinicopathological characteristics (Table 2). In addition, we further to assess the correlation between the expression level of ET-1 and 5-year survival of patients with ESCC using Kaplan-Meier analysis, and the results showed that patients with high ET-1 expression in ESCC tumors displayed a worse overall survival (P<0.05, Figure 1C). Furthermore, RT-qPCR analysis results revealed that the protein level of ET-1 in ESCC cells was higher than in normal esophageal epithelial cells (Het-1A; P<0.001, Figure 1D), especially ECA109 cells (P<0.01). Taken together, ET-1 may serve oncogene in the progression of ESCC.
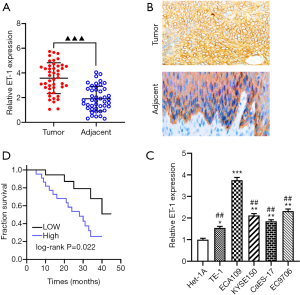
Table 2
| Clinical features | n | ET-1 expression | χ2 | P | |
|---|---|---|---|---|---|
| aLow (%) | High (%) | ||||
| Age (years) | 0.218 | 0.641 | |||
| <60 | 20 | 9 (20.00) | 11 (24.44) | ||
| ≥60 | 25 | 13 (28.89) | 12 (26.67) | ||
| Gender | 0.025 | 0.873 | |||
| Male | 24 | 12 (26.67) | 12 (26.67) | ||
| Female | 21 | 10 (22.22) | 11 (24.44) | ||
| Tumor size (cm) | 5.380 | 0.020 | |||
| <3 | 15 | 11 (24.44) | 4 (8.89) | ||
| ≥3 | 30 | 11 (24.44) | 19 (42.22) | ||
| TNM stage | 5.021 | 0.025 | |||
| I–II | 19 | 13 (28.89) | 6 (13.33) | ||
| III–IV | 26 | 9 (20.00) | 17 (37.78) | ||
| Lymph node metastases | 4.132 | 0.042 | |||
| No | 14 | 10 (22.22) | 4 (8.89) | ||
| Yes | 31 | 12 (26.67) | 19 (42.22) | ||
a, 45 paired of cases of ESCC tissues were divided into groups according to the median expression of ET-1: a high ET-1 expression group (above the median ET-1 expression) and low ET-1 expression group (below the median ET-1 expression).
Knockdown of ET-1 suppressed the proliferation and metastasis of ESCC cells
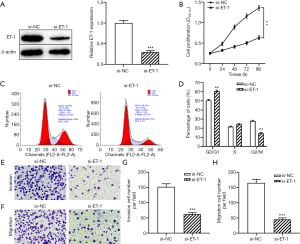
To examine whether ET-1 exhibits tumor-promoting activity through regulating the biological behavior of ESCC cells, ECA109 cells were transfected with ET-1 siRNA to decrease the expression of ET-1. As expected, silencing of ET-1 significantly decreased the protein level of ET-1 in ECA109 cells compared with the control group (P<0.001, Figure 2A). Next, the effect of ET-1 on the proliferation, cell cycle, invasion, and migration of ECA109 cells were examined by CCK-8, flow cytometry and Transwell assay. CCK-8 assay showed that knockdown of ET-1 significantly inhibited the proliferation ability of ECA109 cells compared with the si-NC group (both P<0.01, Figure 2B). Knockdown of ET-1 significantly decreased the number of ECA109 cells in G2/M phase and increased the number of cells in the G0/G1 phase (P<0.01, Figure 2C,D). Moreover, Transwell assay results showed that ET-1 knockdown markedly reduced the migration and invasion capacity of ECA109 cells (both P<0.001, Figure 2E,F,G,H). Taken together, the above results suggested that the downregulation of ET-1 exerted an anti-proliferation and anti-metastasis effect in ESCC.
Knockdown of ET-1 blocked the PI3K/Akt signaling pathway
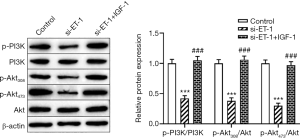
Increasing evidence confirmed that the PI3K/Akt signaling pathway plays an important role in the progression of tumors, including ESCC (30), gastric cancer (31), and lung cancer (32), etc. Herein, to further explore whether ET-1 regulated the progression of ESCC via the PI3K/Akt signaling pathway. As shown in Figure 3, compared with the control group, knockdown of ET-1 significantly decreased the levels of p-PI3K, p-Akt308 and p-Akt473 in ECA109 cells (both P<0.001), while no change the expression of PI3K and Akt. Of note, ECA109 cells treated with the PI3K/Akt signaling pathway activator IGF-1 significantly attenuated the inhibitory effect of ET-1-silenced on the expression of p-PI3K, p-Akt308 and p-Akt473 (both P<0.001). Taken together, knockdown of ET-1 inactivated the PI3K/Akt signaling pathway in ESCC cells.
Silencing of ET-1 inhibited proliferation and metastasis of ESCC cells through inactivating the PI3K/Akt signaling pathway
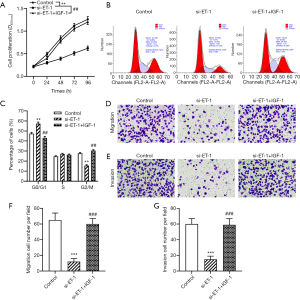
To further examine whether ET-1-silenced serves anti-proliferation and anti-metastasis activities in ESCC through inhibition of the PI3K/Akt signaling pathway. As shown in Figure 4A, treatment with PI3K/Akt signaling pathway activator IGF-1 plus knockdown of ET-1 significantly alleviated the inhibitory effect of ET-1-silenced on the proliferation ability of ECA109 cells (P<0.01). Moreover, compared with the si-ET-1 group, the number of G2/M phase and G0/G1 phase in ECA109 cells transfected with ET-1 siRNA and then treated with IGF-1 were significantly decrease and increased (all P<0.01, Figure 4B,C). Furthermore, we examined whether IGF-1 could downregulate the effect of ET-1 knockdown on the invasion and migration capacity of ESCC cells. As expected, IGF-1 treatment blocked the knockdown of ET-1 suppressed the invasion and migration of ECA109 cells (both P<0.001, Figure 4D,E,F,G). Of note, no significant difference between the control group and the si-ET-1 + IGF-1 group. The results showed that the activation of the PI3K/Akt signaling pathway could alleviate the inhibitory effect of ET-1-silenced on the proliferation and metastasis effect in ESCC cells.
ET-1 inhibition suppressed tumor growth of ESCC in vivo
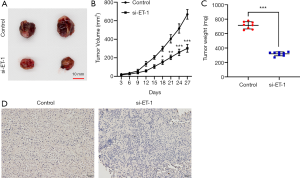
To further measure the effect of inhibition of ET-1 on tumor growth of ESCC in vivo. We observed that knockdown of ET-1 resulted in a significantly decreased tumor growth (Figure 5A,B) and tumor weight (P<0.001, Figure 5C) when compared with the control group. Immunohistochemistry results showed that inhibition of ET-1 markedly decreased the level of Ki-67 in tumor tissues (Figure 5D). Taken together, knockdown of ET-1 significantly inhibited tumor growth in vivo.
Discussion
ESCC was a is a common malignant tumor, which has the characteristics of atypical symptoms, difficult treatment and low survival rate (33). However, current treatment methods and effective diagnosis target of ESCC are limited, leading to the poor overall survival of ESCC. In the present study, we demonstrated that ET-1 plays an important role in the progression of ESCC, inhibition of ET-1 significantly decreased cell proliferation, migration, and invasion of ESCC. Mechanistically, ET-1 exhibits anti-proliferation and anti-metastasis activities via blocking the PI3K/Akt signaling pathway in ESCC.
Increasing evidence confirmed that TRE regulated tumor progression through mediating the malignant biological behavior of cancer cells (34). Importantly, some studies have explored the therapeutic targeting of TRE in multiple cancers, and various pharmaceutical inhibitors targeting TRE have been developed (35,36). For example, ET-1, also called endothelin-1, plays a critical role in the progression and development of cancers, including nasopharyngeal carcinoma (37), gastric cancer (38), and prostate cancer (39), while the role and mechanism of ET-1 in ESCC are unknown. In the present study, our data showed that ET-1 was upregulated in tumor tissues and cell lines of ESCC, silencing of ET-1 inhibited cell proliferation and metastasis in ESCC. Moreover, previous studies have confirmed that overexpression of ET-1 promoted the risk of development and progression of papillary thyroid cancer (40) and breast cancer (41). Furthermore, ET-1-elevated induced the migration and proliferation of tumor cells through regulating the PI3K/ERK/c-FOS/AP1 signaling pathway (42). In this study, we demonstrated that inhibition of ET-1 suppressed ESCC cell proliferation, migration and invasion by blocking the PI3K/Akt signaling pathway.
Accumulated studies have explored the therapeutic targeting of the PI3K signaling pathway in various tumors; however, the clinical effect was not satisfactory (43). For example, inhibition of the PI3K/Akt signaling pathway suppressed tumor progression by decreasing cancer stem-like cell expansion (44). Philip et al. showed that the inactivation of the PI3K/Akt signaling pathway improved the individualized treatment effect of endometrial cancer patients with PTEN mutations (45). Ao et al. reported that inhibition of the PI3K/Akt singaling pathway suppressed gastric cancer cell proliferation, migration, and invasion and induced cell apoptosis (46). Liu et al. confirmed that the treatment of hepatocellular carcinoma cells with the PI3K/Akt signaling pathway inhibitor leads to the expansion of liver cancer stem cells via the inactivation of SGK3 (47). In addition, previous studies have proved that the PI3K/Akt signaling pathway serves an important role involved in the process of EMT in various tumors (48). For instance, one of the current studies revealed that inactivation of the PI3K/Akt signaling pathway decreased the epithelial-mesenchymal transition in ovarian cancer (48). In line with the above results, our studies found that activation of the PI3K/Akt signaling pathway induced by IGF-1 addition promoted ESCC cell proliferation, migration, and invasion, while knockdown of ET-1 alleviated this effect.
In conclusion, we have demonstrated that ET-1 plays a vital role in the progression of ESCC. More importantly, treatment with the PI3K/Akt signaling pathway activator IGF-1 alleviated the inhibitory effect of ET-1 knockdown on tumor growth and metastasis of ESCC cells, which is an attractive therapeutic regimen for the treatment of ESCC.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.26). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients selected for our study were fully informed about our experimental protocols, which were approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University (approval ID: 20180528).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lin L, Lin DC. Biological significance of tumor heterogeneity in esophageal squamous cell carcinoma. Cancers (Basel) 2019;11:1156. [Crossref] [PubMed]
- Yang X, Chen X, Zhuang M, et al. Smoking and alcohol drinking in relation to the risk of esophageal squamous cell carcinoma: A population-based case-control study in China. Sci Rep 2017;7:17249. [Crossref] [PubMed]
- di Pietro M, Canto MI, Fitzgerald RC. Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the Esophagus: Screening, Diagnosis, and Therapy. Gastroenterology 2018;154:421-36. [Crossref] [PubMed]
- Yan F, Pidayi M, Xia Y, et al. The prognosis value of C-reactive protein and endothelin-1 in chronic obstructive pulmonary disease patients with pulmonary artery pressure. Pak J Pharm Sci 2019;32:1697-701. [PubMed]
- Zheng LH, Liu SY, Hu F, et al. Association between big endothelin-1 and CHADS2/CHA2DS2-VASc scores in non-valvular atrial fibrillation. J Geriatr Cardiol 2019;16:812-7. [PubMed]
- Rafnsson A, Matic LP, Lengquist M, Mahdi A, et al. Endothelin-1 increases expression and activity of arginase 2 via ETB receptors and is co-expressed with arginase 2 in human atherosclerotic plaques. Atherosclerosis 2020;292:215-23. [Crossref] [PubMed]
- Sin A, Tang W, Wen CY, et al. The emerging role of endothelin-1 in the pathogenesis of subchondral bone disturbance and osteoarthritis. Osteoarthritis Cartilage 2015;23:516-24. [Crossref] [PubMed]
- Tocci P, Caprara V, Cianfrocca R, et al. Endothelin-1/endothelin A receptor axis activates RhoA GTPase in epithelial ovarian cancer. Life Sci 2016;159:49-54. [Crossref] [PubMed]
- Asgari M, Eftekhar E, Abolhasani M, et al. Endothelin-1 Expression in Prostate Needle Biopsy Specimens Correlated With Aggressiveness of Prostatic Cancer. Iran J Pathol 2017;12:171-6. [PubMed]
- Chen CC, Chen LL, Hsu YT, et al. The endothelin-integrin axis is involved in macrophage-induced breast cancer cell chemotactic interactions with endothelial cells. J Biol Chem 2014;289:10029-44. [Crossref] [PubMed]
- León J, Casado J, Jimenez Ruiz SM, et al. Melatonin reduces endothelin-1 expression and secretion in colon cancer cells through the inactivation of FoxO-1 and NF-kappabeta. J Pineal Res 2014;56:415-26. [Crossref] [PubMed]
- Zhang ZY, Chen LL, Xu W, et al. Effects of silencing endothelin-1 on invasion and vascular formation in lung cancer. Oncol Lett 2017;13:4390-6. [Crossref] [PubMed]
- Said N, Smith S, Sanchez-Carbayo M, et al. Tumor endothelin-1 enhances metastatic colonization of the lung in mouse xenograft models of bladder cancer. J Clin Invest 2011;121:132-47. [Crossref] [PubMed]
- Zang X, Zhou Y, Huang Z, et al. Endothelin-1 single nucleotide polymorphisms and risk of pulmonary metastatic osteosarcoma. PLoS One 2013;8:e73349. [Crossref] [PubMed]
- Zhang Y, Yan H, Xu Z, et al. Molecular basis for class side effects associated with PI3K/AKT/mTOR pathway inhibitors. Expert Opin Drug Metab Toxicol 2019;15:767-74. [Crossref] [PubMed]
- Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin Cancer Biol 2019;59:125-32. [Crossref] [PubMed]
- Zhang JJ, Xu WR, Chen B, et al. The up-regulated lncRNA DLX6-AS1 in colorectal cancer promotes cell proliferation, invasion and migration via modulating PI3K/AKT/mTOR pathway. Eur Rev Med Pharmacol Sci 2019;23:8321-31. [PubMed]
- Chen M, Zhu LL, Su JL, et al. Prucalopride inhibits lung cancer cell proliferation, invasion, and migration through blocking of the PI3K/AKT/mTOR signaling pathway. Hum Exp Toxicol 2020;39:173-81. [Crossref] [PubMed]
- Tang G, Tang Q, Jia L, et al. TROP2 increases growth and metastasis of human oral squamous cell carcinoma through activation of the PI3K/Akt signaling pathway. Int J Mol Med 2019;44:2161-70. [PubMed]
- Huang DH, Jian J, Li S, et al. TPX2 silencing exerts antitumor effects on hepatocellular carcinoma by regulating the PI3K/AKT signaling pathway. Int J Mol Med 2019;44:2113-22. [PubMed]
- Ma S, Liu T, Xu L, et al. Histone deacetylases inhibitor MS-275 suppresses human esophageal squamous cell carcinoma cell growth and progression via the PI3K/Akt/mTOR pathway. J Cell Physiol 2019;234:22400-10. [Crossref] [PubMed]
- Zuo WN, Zhu H, Li LP, et al. MiR-155 promotes proliferation and inhibits apoptosis of nasopharyngeal carcinoma cells through targeting PTEN-PI3K/AKT pathway. Eur Rev Med Pharmacol Sci 2019;23:7935-42. [PubMed]
- Carnero A, Blanco-Aparicio C, Renner O, et al. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets 2008;8:187-98. [Crossref] [PubMed]
- Wang H, Yang X, Guo Y, et al. HERG1 promotes esophageal squamous cell carcinoma growth and metastasis through TXNDC5 by activating the PI3K/AKT pathway. J Exp Clin Cancer Res 2019;38:324. [Crossref] [PubMed]
- Wei X, Jia Y, Lou H, et al. Targeting YAP suppresses ovarian cancer progression through regulation of the PI3K/Akt/mTOR pathway. Oncol Rep 2019;42:2768-76. [PubMed]
- D'Antoni S, Ranno E, Spatuzza M, et al. Endothelin-1 induces degeneration of cultured motor neurons through a mechanism mediated by nitric oxide and PI3K/Akt pathway. Neurotox Res 2017;32:58-70. [Crossref] [PubMed]
- Horowitz JC, Ajayi IO, Kulasekaran P, et al. Survivin expression induced by endothelin-1 promotes myofibroblast resistance to apoptosis. Int J Biochem Cell Biol 2012;44:158-69. [Crossref] [PubMed]
- Niu Y, Zhang J, Tong Y, et al. Physcion 8-O-beta-glucopyranoside induced ferroptosis via regulating miR-103a-3p/GLS2 axis in gastric cancer. Life Sci 2019;237:116893. [Crossref] [PubMed]
- Shi L, Zhou SS, Chen WB, et al. Functions of endothelin-1 in apoptosis and migration in hepatocellular carcinoma. Exp Ther Med 2017;13:3116-22. [Crossref] [PubMed]
- Wang L, Zhang Z, Yu X, et al. SOX9/miR-203a axis drives PI3K/AKT signaling to promote esophageal cancer progression. Cancer Lett 2020;468:14-26. [Crossref] [PubMed]
- Lin JX, Xie XS, Weng XF, et al. UFM1 suppresses invasive activities of gastric cancer cells by attenuating the expres7sion of PDK1 through PI3K/AKT signaling. J Exp Clin Cancer Res 2019;38:410. [Crossref] [PubMed]
- Zhang N, Yan QQ, Lu L, et al. The KLF6 splice variant KLF6-SV1 promotes proliferation and invasion of non-small cell lung cancer by up-regultating PI3K-AKT signaling pathway. J Cancer 2019;10:5324-31. [Crossref] [PubMed]
- Saemi N, Khoshnevis J, Akbari ME, et al. Evaluating the Correlation Between the Survival Rate of Patients with Esophageal Squamous Cell Carcinoma and Expression of p53 and Cyclin D1 Biomarkers Along with Other Prognostic Factors. J Gastrointest Cancer 2018;49:35-40. [Crossref] [PubMed]
- Qu Y, Liu L, Niu Y, et al. Viral proliferation and expression of tumor-related gene in different chicken embryo fibroblasts infected with different tumorigenic phenotypes of avian leukosis virus subgroup Poult Sci 2016;95:2383-90. [Crossref] [PubMed]
- Zhou C, Wang P, Tu M, et al. DEPDC1 promotes cell proliferation and suppresses sensitivity to chemotherapy in human hepatocellular carcinoma. Biosci Rep 2019;39:BSR20190946. [Crossref] [PubMed]
- Chen W, Liu H, Wang T, et al. Downregulation of AIF-2 Inhibits Proliferation, Migration, and Invasion of Human Glioma Cells via Mitochondrial Dysfunction. J Mol Neurosci 2019;68:304-10. [Crossref] [PubMed]
- Yin P, Song G, Jiang Z. Cisplatin suppresses proliferation, migration and invasion of nasopharyngeal carcinoma cells in vitro by repressing the Wnt/beta-catenin/Endothelin-1 axis via activating B cell translocation gene 1. Cancer Chemother Pharmacol 2018;81:863-72. [Crossref] [PubMed]
- Tsai KW, Hu LY, Chen TW, et al. Emerging role of microRNAs in modulating endothelin-1 expression in gastric cancer. Oncol Rep 2015;33:485-93. [Crossref] [PubMed]
- Xu D, Wang X, Lou Y. Association of endothelin-1 gene single-nucleotide polymorphisms and haplotypes with risk of hormone refractory prostate cancer. Pharmazie 2017;72:103-6. [PubMed]
- Aydin AF, Vural P, Dogru-Abbasoglu S, et al. The endothelin 1 and endothelin receptor A gene polymorphisms increase the risk of developing papillary thyroid cancer. Mol Biol Rep 2019;46:199-205. [Crossref] [PubMed]
- Gampenrieder SP, Hufnagl C, Brechelmacher S, et al. Endothelin-1 genetic polymorphism as predictive marker for bevacizumab in metastatic breast cancer. Pharmacogenomics J 2017;17:344-50. [Crossref] [PubMed]
- Bartella V, De Francesco EM, Perri MG, et al. The G protein estrogen receptor (GPER) is regulated by endothelin-1 mediated signaling in cancer cells. Cell Signal 2016;28:61-71. [Crossref] [PubMed]
- Yang J, Nie J, Ma X, et al. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer 2019;18:26. [Crossref] [PubMed]
- Ioris RM, Galie M, Ramadori G, et al. SIRT6 Suppresses Cancer Stem-like Capacity in Tumors with PI3K Activation Independently of Its Deacetylase Activity. Cell Rep 2017;18:1858-68. [Crossref] [PubMed]
- Philip CA, Laskov I, Beauchamp MC, et al. Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer 2017;17:638. [Crossref] [PubMed]
- Ao R, Guan L, Wang Y, et al. Silencing of COL1A2, COL6A3, and THBS2 inhibits gastric cancer cell proliferation, migration, and invasion while promoting apoptosis through the PI3k-Akt signaling pathway. J Cell Biochem 2018;119:4420-34. [Crossref] [PubMed]
- Liu F, Wu X, Jiang X, et al. Prolonged inhibition of class I PI3K promotes liver cancer stem cell expansion by augmenting SGK3/GSK-3beta/beta-catenin signalling. J Exp Clin Cancer Res 2018;37:122. [Crossref] [PubMed]
- Liu L, Wu N, Wang Y, et al. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K/AKT oncogenic signaling. J Exp Clin Cancer Res 2019;38:106. [Crossref] [PubMed]



