
Endometrial cancer—is our knowledge changing?
Introduction
Endometrial cancer (EC) arising from the uterine endometrium is currently the fourth most common cancer in women with ever increasing incidence, particularly in the last decade (1). Among gynecologic cancers, it is the most common one (2) with over 380,000 new patients every year. In addition, a robust escalation by up to a 100% in the next 5 years is predicted (3) and survival has not changed in the last decade. Despite these facts, from the global point of view, EC somehow is a neglected disease.
On the positive side, the majority of patients have low-grade endometrioid cancer with a 5-year survival rate of 83% (4). The opposite situation occurs in case of high-risk EC with rather low survival rates (5). In women with either advanced or relapsed cancer, the prognosis is dismal. In addition to these two most common EC types, rare neuroendocrine and undifferentiated tumors belong to the EC designation. Both types of EC show strong disparity due to race with significantly lower survival rates for white women compared to black women (83% vs. 62%) (6).
Another way of classifying EC is based on the idea of Borhman (in 1983), who suggested the existence of two significantly different types of EC: more common ER+ type I and less common ER− type II. As ER-targeted therapy was found to be successful, endocrine therapy is sometimes useful in EC too (7). EC can, however, change from type I to type II, making the estrogen therapy worthless (8). Further studies focused on estrogen-related receptor (ERR) α, which can be a valuable addition, particularly when a dual targeting or ERRα and ERα is used (9). Some recent studies proposed a novel subtype developing directly from normal endometrium. Analysis of genomic data further divided this type of EC into two subgroups which developed under completely different histopathological mechanisms (10).
On the molecular level, EC is relatively well-studied. These cancers are characterized by frequent changes in the P13K-AKT-mTOAR, RAS-RAF-MAPK-ERK, and WNT/β-catenin pathway with relatively high microsatellite instability (11). Newer studies focused on dysregulated gene expression using next generation sequencing and bioinformatics. A comparison of EC and normal tissue revealed 56 dysregulated genes (9 downregulated and 47 upregulated). The association of these genes varied from immune system action to stimuli responses (Figure 1). Some of these genes were associated with cellular movement and cell death. Six of these genes were strongly connected with poor prognosis, three of them were connected with good prognosis. The authors followed up with combining these data with micro-RNA-mediated gene expression and found two strongly dysregulated association—hsa-miR-218-5p associated with downregulated HPGD and hsa-mIR-127-5p with upregulated CSTB. How these findings can be transformed into either diagnosis or therapies of EC remains unclear.
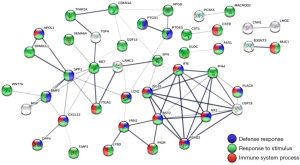
Many studies suggested the role of changes in gene expression and regulation in EC development. One of these changes involve suppressed expression of LncRNA-FER1L4 possibly leading to the suppressed proliferation of EC cells (13). Another potentially important gene is transformer 2 protein homolog beta (TRA2B), which is also involved in breast, lung and cervical cancer (14,15), most probably via regulation of carcinogenesis and viability of cancer cells (16). A recent study confirmed the regulatory role of TRA2B in EC with overexpression of TRA2B clearly correlating with increased proliferation of EC cells. In addition, inhibition of TRA2B expression by siRNA reversed these effects and increased apoptosis (17), suggesting not only prognostic role of TRA2B expression, but also making this gene a potential target for development of EC treatment. Another possibility is the presence of SOX17. Recent studies found that downregulation of SOX17 results in facilitation of epithelial-mesenchymal transition via regulation of β-catenin expression and Wnt signaling (18).
Endometriosis is generally manifested by the abnormal presence of both endometrial glandular and stromal cells outside the uterine cavity. The complex pathogenesis of this disease remains controversial despite decades of extensive research, resulting in relatively little progress in treatment. The pathogenesis of endometriosis has been the focus of attention of long and active investigations, and numerous hypotheses have been reached, unfortunately without a general consensus [see review by Vetvicka and Kralickova (19)].
Capability of endometriosis to undergo malignant transformation is well established. However, despite long and intensive research, we still cannot identify definite intermediate precursors. The closest example might be an atypical endometriosis with features that are neither benign nor fully malignant (20).
Differences between low- and high-risk EC
Based on numerous factors such as epidemiological, endocrine and clinical findings, EC is usually determined as low-grade (type I) and high-grade (type II) (21). Almost 40 years later, numerous studies suggested that this nomenclature is not perfect (22). From the clinical point of view, both types substantially differ from each other. Low-risk EC usually occurs in young women and in peri-menopausal women diagnosed previously with proliferative endometrium of endometrial hyperplasia. On the other hand, high-risk EC appearing in postmenopausal patients, are characterized with atrophic endometrium and have no relation to excessive estrogen levels or obesity.
Low-risk EC clearly results from endometrial hyperplasia known for high levels of estrogen. This serves as an immediate precursor lesion for EC. DNA errors, probably caused by excessive proliferation, might support the progression from low-grade to high-grade EC.
High-risk EC starts with anthropic endometrium, but the mechanisms of changing of endometrium into this relatively rare cancer type are generally unknown. Histological classification revealed some differences in markers (8% vs. 33–46% for ARID1A, 39% vs. 5% for HNF1β, 3–18% vs. 18–69% for p53, and 0–3% vs. 0–27% for WT1), suggesting the need for different treatments, but not offering any information about the mechanisms of EC development (2). For comprehensive review of these two types of EC (2).
Risk factors
Numerous risk factors have been found to affect the EC, including age, obesity (23), nulliparity (24), early menarche (25), infertility, family history (26) and hormone replacement therapy (27). Among stronger risks is endometrial hyperplasia [for review, see Kralickova et al. (28)]. Other studies mentioned hypertension and diabetes, both associated with chronic inflammation. As it might participate in EC development and progression, expression of NLRP3 inflammasome was studied (29). The upregulation of this inflammasome by estrogen via ERβ was found to increase the progression of EC, making NLRP3 inflammasome a potential therapeutic target. On the other hand, expression of ERβ has inversed role in EC progression, with downregulated ERβ associated with elevated progression, making inflammasome somehow questionable.
Recently, nutrition became a target of numerous studies trying to find a correlation between nutrition or nutritional habits and various diseases including cancer. A meta-analysis of 16 studies revealed clear inverse association between EC and consumption of dietary fiber (30). However, the effects of increased fiber consumption on lowering of EC risk seem to be only low.
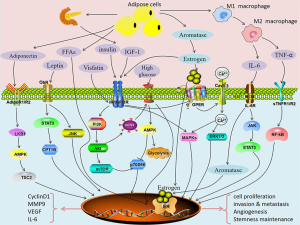
Obesity is generally considered to be one of the main risk factors for EC development. So far, the only available option was the recommendation to lose weight. Recently, the in vitro and in vivo study of the effects of metformin (a known drug for treatment of metabolic syndrome) employed a novel mouse model of EC under both lean and obese conditions. This drug inhibited EC growth in both lean and obese animals, but the degree of inhibition was significantly higher in obese mice. The reasons for the better effects in obese animals might lie in different metabolomic profiles in EC tissue with much higher energy metabolism, lipid peroxidation and lipid biosynthesis observed in obese animals (31). The mechanism of action might be the reversion of detrimental metabolic effects. However, a recent Brazilian study found no correlation between obesity and prognosis of EC (32), making the older, well-established hypotheses rather questionable. The reason might be the fact that in many cases, the higher mortality in obese groups results more from morbid obesity than from EC. At the same time, studies like these clearly demonstrate the problems with older, supposedly iron clad hypothesis on EC. Due to these inconsistent results, it might be better to focus on the possible role of metabolic syndrome, which involves obesity, high blood pressure and sugar, high triglycerides and low HDL levels. At least three parts of the metabolic syndrome—diabetes, hypertension and obesity—are present in most cases of EC, but no mechanisms connecting these conditions to the genesis and development of EC are known. Numerous metabolic and signaling pathways and biomarkers expression (inflammation, HbA1c, CRP, leptin, adiponectin, BMI-sensitive pathway of insulin resistance) have been suggested (Figure 2) (33), but this data offers more information overkill than a clear cut marker or mechanism.
Some research into risk factors involved in EC development and/or progression seems to be more a fishing expedition than a well thought-out approach. As an example, many serve findings showing that papillomavirus presence is an independent risk factor (34). As the study was performed in only one country, it has limited value. In addition, with an approximate 25% infection rate throughout the world, human papillomavirus represents a questionable biomarker.
The presence of glandular cells was found to be another risk factor for EC. A large retrospective study found strong correlation between the local recurrence of EC and the presence of glandular cells (35).
Markers
As current information does not allow precise types of treatment, the search for potential markers suitable for development into pharmaceuticals is ongoing. This process is complicated by the fact that EC can be classified into numerous types. While most of EC are carcinomas, sarcomas are not rare. In addition, histological classification recognized endometroid, clear cell and serous EC [for details on classification, see Urick et al. (36)]. As individual types of EC differ in molecular characteristics, it is understandable that the high amount of possible markers make even the evaluation of possible clinical potential difficult, and development into attainable treatment impossible. Thirteen most common somatic aberration frequencies in individual types and subtypes of EC found no clear cut differences among individual types, but also no clear yes or no answer regarding prognosis or potential treatment (35).
One potential marker might be long non-coding RNAs, because they act as molecular based classification (37). As our knowledge of biological functions of long non-coding RNAs is still in the preliminary stage, this hypothesis needs to be further documented. However, this research continues despite these setbacks, resulting in creation of ceRNA network of EC with the aim of identifying prognostic biomarkers. So far, twelve possible biomarkers were identified (38).
Recently, voltage-gated Na channel Nav1.7 has been proposed as a promising marker useful for both prognosis and treatment of EC (39). However, our knowledge is so far based entirely on in vitro experiments.
Keratin 17 was recently found to be a negative prognostic marker in high-grade EC, as the expression in malignant glandular cells of endometrium corresponded with lower survival rates (40). This finding was not surprising, as keratin 17 was previously found to be a negative prognostic marker in numerous cancers including cervical carcinoma, some ovarian cancers and pancreatic carcinoma (41). It is surprising that despite all this knowledge, keratin 17 was never tested as a pharmacologic target.
Another possible marker might be agglutinin proteins Galectin-1 and Galectin-9. These proteins were previously found expressed in numerous tumors and play some role in cancer formation (42). In EC, Galectin-1 was associated with poor prognosis and Galectin-9 expression corresponded to early pathological changes (43). However, similar to most EC studies, uneven distribution of cases and low number of patients result in doubts about the significance of these results.
A novel approach to identification of possible biomarkers is the use of NMR spectroscopy. Using a model of AI algorithms, a screening of 54 women with potential EC found that the presence of asparagine, phosphocholine, and malate in cervicovaginal fluid might represent a potential metabolomic biomarker (44). Although, to propose usage of possible markers based on only one study with a clearly insufficient number of patients is rather impossible.
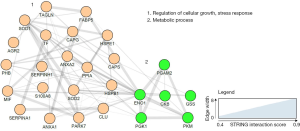
A potentially important approach is using proteomics, as these techniques identify molecular fingerprints via evaluation of protein pathways and characterize all protein markup inside the cell, also their modifications and possible interactions. Using these techniques, several tissue- and blood-based potential biomarkers (Figure 3) have been described (45), but their use in clinical practice is still far off.
Despite long interests and significant effort, there are no validated markers, particularly for early detection of EC.
Prognosis
FIGO staging (International Federation of Gynecology and Obstetrics staging), cervical involvement, differentiation grades and myometrial infiltration are the major prognostic features (46). Another classification is based on combined analysis of somatic copy number alterations, mutation burden and microsatellite instability. Based on this classification, EC can be divided into four groups: POLE ultramutated, microsatellite instability hypermutated, copy number low and copy number high (47). Ultrasonic measurements of endometrial thickness is a solid predictor of EC, but cannot distinguish histologic subtypes and do not correlate to FIGO stages (48).
Another important prognostic factor is lymph node metastasis (4), which is based on the landmark GOG 33 trial. Complete lymphadenectomy has been routinely suggested, but later analysis found that in EC with grade 1 or 2 and below 2 cm in diameter, there are no lymph node metastases (49). This suggests the need of lymph node removal only in patients with later stages of EC. Since then, the question whether the complete lymphadenectomy should not be performed or whether it is a valid treatment is still open and fiercely debated (50). More and more studies suggest the possible end of comprehensive lymphadenectomy.
Computed tomography (CET/PT) is often used for prediction of nodal status prior surgical procedures, but the sensitivity reaching 53% needs to be improved (51). New studies, however, found much better results and demonstrated high specificity, allowing the recommendation of CET/PT for staging and detection of distant metastases (52).
Besides individual risks factors, surgical classification is routinely used for prognostic evaluations. However, no standardization or guidelines suggesting the relation between stages and treatment management exist. A detailed study comparing various parameters found that metabolic tumor volume and total lesion glycolysis serve as strong prognostic factors (53). With relatively low numbers of patients, this study needs independent confirmation. It is interesting that tumor size has no prognostic value, at least in patients with stage I or II of EC (54). Nonetheless, this study excluded extensive amounts of patients including those with extrauterine spread and those not having lymphadenectomy. This severely limited the value of these findings. A retrospective study evaluated potential prognostic factors in 874 patients receiving surgery and found that only cervical invasion, ovarian metastasis and lymphopoieses space invasion clearly correlated with the risk of lymph node metastasis (55).
A past study of 690 women treated in one hospital during a 2006–2017 period found that only age, stage and BMI are independent prognostic factors (56). In high-risk patients, the risk assessment did not provide any clear information (57). However, it is important to keep in mind that this study is continuing and the main purpose was to collect specimens for future evaluation.
Another former study compared intraoperative and preoperative staging and concluded that the highest specificity was achieved with intraoperative screening of frozen sections, although the differences were minimal (58). Recently, liquid biopsy has become an important prognostic tool offering important information on the genetic basis of EC. A 5-year follow-up study found the optimal techniques for pre-surgery evaluation of prognostic factors are hysteroscopic excisional biopsy and MRI with a specificity over 88% (59). The significant value of MRI was further confirmed by a meta-analysis of 14 studies (60). Another possible predictive marker of the local recurrence of EC might be presence of glandular cells, found in an historical cohort study of patients treated in one hospital from 1990 to 2012 (35). If confirmed, this information might be not only important for prediction of recurrence, but also offering an easy prediction, as cytologic examination is routinely done before surgery.
MicroRNAs (miRNAs) represent short non-coding single-stranded RNA with significant roles in numerous biological processes including cancer. miRNA-103 was found to be strongly upregulated in EC and to significantly stimulate proliferation of EC cells. Further studies suggested an inverse correlation between miRNA-103 and ZO-1. Downregulation of ZO-1 resulted in increased cell proliferation, whereas downregulation of miRNA-103 suppressed cell proliferation (61). Despite this correlation, we do not know if miRNA-103 really acts through ZO-1.
One of the new approaches to future treatment is based on function of long non-coding RNAs (lncRNAs), which are longer than 200 nucleotides RNA transcripts which do not encode proteins but have wide regulatory roles in many biological processes including cancer development (62). Out of many, lncRNA nuclear enriched abundant transcript 1 (NEAT1) was found the most dysregulated in wide range of tumors including colorectal, bladder and breast cancer (63). In EC, it stimulates proliferation, migration and cell invasion via regulation of the miR-144-3p/EZH2 axis (64). As the NEAT1 can be knocked down, it might offer a potential target for suppression of EC invasion.
Another prognostic factor can be the presence of somatic POLE exonuclease domain mutation. Recent studies, however, suggested caution in using this mutation for prognosis of EC, as the results were not clear and were difficult to interpret (65).
An interesting finding of possible negative effects of tamoxifen treatment of breast cancer patients revealed the incidence of EC in breast cancer patients is significantly elevated, most probably due to the tamoxifen therapy. This trend was observed in both ER+ and HR− patients (66). This suggests that the hormone receptor status is not important.
Enhancer of zeste homolog 2 (EZH2) is involved in regulation of transcription. With increased expression in various tumors, EZH2 was suggested to be a promising candidate for targeted therapy in numerous tumors (66) including prostate, breast and GI tumors. A recent study of the role of EZH2 in EC found strong correlation of disease-free and survival with EXH2 overexpression and specific silencing of EZH2 resulting in enhanced effects of chemotherapy. In addition, this EZH2 silencing down-regulated expression of numerous tumor-associated genes such as PRDX6 (67), suggests that this treatment might be suitable for gynecological cancers too.
Kallikrein-related peptidase (KLK) family was, in addition to other cancer types, studied in EC. Analysis of mRNA revealed that high expression of KLK5-8 was significantly associated with aggressive phenotype of EC and with worse prognosis (68), making KLK family both biomarker and prognostic factor.
An additional prognostic factor associated with poor outcome is the presence of lymphovascular space invasion. However, the impact of this knowledge on the treatment and subsequent prognosis is unknown and a detailed quantitation of lymphovascular space invasion as a base for decision on treatment is recommended (69).
Using the cluster analysis of data from The Cancer Genome Atlas research network (47), a recent study hypothesized that direct molecular characterization of EC will reveal mechanisms resulting in failure of treatment. For detailed cluster analysis and for attempts for integration of molecular and clinical characteristics into prediction models [see Miller et al. (70)].
Treatment
The standard treatment of all types of EC consists of a combination of surgery, irradiation and/or chemotherapy, with all three options having some negative side effects. In general, there is no consensus on an optimal treatment, which is particularly true in case of treatments optimized for individual stages of EC. Adjuvant therapy is sometimes used, but no consensus on the optimal treatment has been found and this treatment often offers unsatisfactory results (71). On the other hand, hormonal therapy, particularly using anti-estrogens and progesterone, is often used in patients requesting preservation of their fertility (72), patients with recurrent problems or unable to undergo surgery. Gene and immune therapy are more often used for serous EC with overexpression of ERBB2 (73). A recent review tried to compare different therapies and suggested that the first choice of the early stages is surgery, whereas chemotherapy and/or radiation is better for later stages (74). For a detailed comparison of various targeted therapies [see Lheureux et al. (75)]. A comparative study of EC management in elderly patients found the need for using identical treatment used for younger patients (76). Despite the decades of research and treatment, we are still not sure if early chemotherapy really improves survival or not. In other words, what is the appropriate and most advantageous sequence of treatments? Direct trials comparing radiation therapy and chemotherapy failed to yield consistent results (77), most of them suggesting no differences in overall survival rate (78). This study, however, suffered from limitations such as using only Medicare patients (limiting the patients to females older than 64 years) and using different doses among different groups, limiting the impact of the conclusions.
Positron emission tomography-computed tomography was suggested for preoperative management of early stage EC, particularly of the intermediate- and high-risk stage. Low sensitivity for lymph node metastasis limits its utility to be used alone, however it still represents a valuable addition for improvements of diagnosis (79). Independent study did not confirm these results (80), making further utilization of this technique rather questionable.
Among particularly promising ideas are clinical impact evaluation of deregulation among various signaling pathways (e.g., m HER, MAPK or phosphoinositide 3-kinase), but more investigation is necessary.
One of the possibilities are EC stem cells. On one hand, they are, to some extent, involved in all steps of EC development, from progression to metastasis and development of chemoresistance. With the controversial isolation of these cells, their phenotypic and genotypic markup is not clear. Some pathways activated in EC stem cells include Hedgehog, Wingless-INT and Notch1. The selective targeting of these pathways resulted in somewhat encouraging results, for we are still far from clinical studies [for review see Giannone et al. (81)].
An interesting possibility is the use on microRNA inhibition of EC development. Using both in vitro and in vivo experiments, microRNA-23a has been found to block EC via specific targeting sine oculis homeobox homolog 1 (SIX1) (82).
PI3K/AKT/mTOR (PAM) signaling was often suggested as a druggable target for EC, as alterations of this pathway are well documented. PAM inhibitors, however, failed in early clinical trials. In addition to the lack of positive results, these treatments were also suffering from negative side effects. Many patients who received mTOR inhibitors with conventional drugs suffered from higher risk of EC worsening. The situation was opposite when mTOR inhibitors were used after conventional therapy (83). These unclear results obtained from just one study are difficult to interpret. A recent review suggested that tailoring these inhibitors to specific subpopulations might have better results (84), but offer direct proof of this hypothesis.
An interesting approach to EC treatment is to increase the expression of CACNA2D3, which is part of the Ca channel regulatory subunit family linked to tumor suppression. Experimental increase of CACNA2D3 expression resulted in EC cancer inhibition both in vivo and in vitro. Further evaluation revealed that progesterone is involved in changes of CACNA2D3 expression with possible mechanisms being apoptosis promotion via CACNA2D3/Ca2+/p38 MAPK pathway activation (85).
Protocathedrin 10 is sometimes proposed as a tumor suppressor. A finding of protocadherin 10-Wnt-MALAT1 regulatory axis in EC occurred recently (86), with all parts known for involvement in cancer regulation. It might offer a novel approach. With very limited knowledge of this hypothetical mechanism, more studies are clearly needed.
Some studies have found some improvements in patient mortality using statins (87), offering unconventional thought, but additional studies performed in different countries found no association (88). Considering the steady increase in statin use and at the same time increasing incidence of EC, the possible positive correlation between statin use and improved mortality is highly improbable.
Myeloid-derived suppressor cells are involved in EC development via regulation of systemic inflammatory responses. These cells involved both T lymphocytes and stem cells. Their infiltration regulates not only the progression of the disease, but also the sensitivity and resistance to some types of chemotherapy. As their depletion resulted in EC inhibition and increased chemosensitivity, targeting of the myeloid-derived cells can offer a novel type of therapy (89).
A relatively common fraction of EC are cancers with microsatellite instability and mismatch repair deficiency, which can be easily recognized by routine immunohistochemistry (90). Anti-programmed cell death-1 monoclonal antibodies, recently approved by FDA, represent a promising treatment to this new therapy using immune checkpoint inhibition (12). Current knowledge is limited, the microsatellite instability and mismatch repair deficiency is not commonly diagnosed and detailed clinical trials are clearly necessary.
As the current treatment of EC did not significantly improve in the last decades, it is not surprising that various botanicals or medicinal extracts are being evaluated. One of these compounds is wogonoside, a flavonoid component isolated from Scutellaria baicalensis Georgi. The experimental treatment of mice with implanted EC found decreased cell proliferation and metastases, probably via elevating ER stress and subsequent activation of Hippo signaling pathway (44).
However, we are still far from any type of a tailored therapy of EC.
Conclusions
Despite long time interests and extensive research, survival rate of EC has not improved and in many countries is even falling. In addition, rapid escalation is expected to occur in the next decade, mostly due to the fast rise in obesity and longer lifespan.
Novel approaches to the early diagnosis and better research of genetics, risk factors and therapies are needed. Some types of research and treatments are based on the availability of biobanks. Regardless, variations in collection, storage and processing often result in irreproducible data, hampering the progress in development of prognostic, diagnostic and therapeutic advances. So far, no adequate biomarker useful in prognosis of EC is currently available. Similarly, elucidation of the mechanisms how any of the potential biomarkers might influence the genesis and/or progression of EC is still far away. Target-specific treatment of cancer is one of the promises current medicine often gives, but rarely delivers. The common problems involve lack of clinical trials, fragmenting resources on too many marginal targets and lack of novel therapies. In addition, clinical trials targeting some genetic aberrations yielded only modest results. PI3K pathway or ERBB2 in EC can serve as an example (75). Readers interested in the latest review of molecular targets in EC should read (36). The fact that beyond the LHRH linked with doxorubicin (91), no further potential drug reached phase III. This offers little optimism.
On the other hand, there is still a reason for hope. Four molecular subtypes of EC have been recently characterized in substantial details and this classification is already being used in new round of clinical trials. If the molecular classification could be moved up-front, it might provide predictive and even prognostic information which might be much more precise than trying to characterize diverse recurrent EC (92).
With the steadily increasing EC occurrence and the lack of progress in prediction and diagnosis, it seems that the most important part in preventing EC-related death are old fashioned improvements in lifestyle.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Endometrial Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1720). The series “Endometrial Cancer” was commissioned by the editorial office without any funding or sponsorship. Antonio Simone Laganà served as the unpaid Guest Editor of the series, and serves as an unpaid editorial board member of Translational Cancer Research from Dec 2019 to Nov 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lindfors A, Akesson A, Staf C, et al. Robotic vs Open Surgery for Endometrial Cancer in Elderly Patients: Surgical Outcome, Survival, and Cost Analysis. Int J Gynecol Cancer 2018;28:692-9. [Crossref] [PubMed]
- Pandita P, Wang X, Jones DE, et al. Unique Molecular Features in High-Risk Histology Endometrial Cancers. Cancers (Basel) 2019; [Crossref] [PubMed]
- Adishesh M, Hapangama DK. Enriching Personalized Endometrial Cancer Research with the Harmonization of Biobanking Standards. Cancers (Basel) 2019; [Crossref] [PubMed]
- Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987;60:2035-41. [Crossref] [PubMed]
- Vogel TJ, Knickerbocker A, Shah CA, et al. An analysis of current treatment practice in uterine papillary serous and clear cell carcinoma at two high volume cancer centers. J Gynecol Oncol 2015;26:25-31. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Ruiz MP, Huang Y, Hou JY, et al. All-cause mortality in young women with endometrial cancer receiving progesterone therapy. Am J Obstet Gynecol 2017;217:669.e1-e13. [Crossref] [PubMed]
- Kuukasjarvi T, Kononen J, Helin H, et al. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol 1996;14:2584-9. [Crossref] [PubMed]
- Mao X, Dong B, Gao M, et al. Dual targeting of estrogen receptor alpha and estrogen-related receptor alpha: a novel endocrine therapy for endometrial cancer. Onco Targets Ther 2019;12:6757-67. [Crossref] [PubMed]
- Sugiyama Y, Gotoh O, Fukui N, et al. Two Distinct Tumorigenic Processes in Endometrial Endometrioid Adenocarcinoma. Am J Pathol 2020;190:234-51. [Crossref] [PubMed]
- O'Hara AJ, Le Gallo M, Rudd ML, et al. High-resolution copy number analysis of clear cell endometrial carcinoma. Cancer Genet 2020;240:5-14. [Crossref] [PubMed]
- Sobecki-Rausch J, Barroilhet L. Anti-programmed Death-1 Immunotherapy for Endometrial Cancer with Microsatellite Instability-High Tumors. Curr Treat Options Oncol 2019;20:83. [Crossref] [PubMed]
- Qiao Q, Li H. LncRNA FER1L4 suppresses cancer cell proliferation and cycle by regulating PTEN expression in endometrial carcinoma. Biochem Biophys Res Commun 2016;478:507-12. [Crossref] [PubMed]
- Gabriel B, Zur Hausen A, Bouda J, et al. Significance of nuclear hTra2-beta1 expression in cervical cancer. Acta Obstet Gynecol Scand 2009;88:216-21. [Crossref] [PubMed]
- Watermann DO, Tang Y, Zur Hausen A, et al. Splicing factor Tra2-beta1 is specifically induced in breast cancer and regulates alternative splicing of the CD44 gene. Cancer Res 2006;66:4774-80. [Crossref] [PubMed]
- Munkley J, Livermore K, Rajan P, et al. RNA splicing and splicing regulator changes in prostate cancer pathology. Hum Genet 2017;136:1143-54. [Crossref] [PubMed]
- Paudel D, Ouyang Y, Huang Q, et al. Expression of TRA2B in endometrial carcinoma and its regulatory roles in endometrial carcinoma cells. Oncol Lett 2019;18:2455-63. [Crossref] [PubMed]
- Zhou W, Wang K, Wang J, et al. SOX17 Inhibits Tumor Metastasis Via Wnt Signaling In Endometrial Cancer. Onco Targets Ther 2019;12:8275-86. [Crossref] [PubMed]
- Vetvicka V, Kralickova M. Current theories on endometriosis pathogenesis. Edorium J Mol Pathol 2015;1:1-4.
- LaGrenade A, Silverberg SG. Ovarian tumors associated with atypical endometriosis. Hum Pathol 1988;19:1080-4. [Crossref] [PubMed]
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 1983;15:10-7. [Crossref] [PubMed]
- Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol 2014;15:e268-78. [Crossref] [PubMed]
- Januszek SM, Barnas E, Skret-Magierlo J, et al. Obesity as a risk factor of in-hospital outcomes in patients with endometrial cancer treated with traditional surgical mode. Ginekol Pol 2019;90:549-56. [Crossref] [PubMed]
- Pearson-Stuttard J, Zhou B, Kontis V, et al. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol 2018;6:e6-e15. [Crossref] [PubMed]
- Xu WH, Xiang YB, Ruan ZX, et al. Menstrual and reproductive factors and endometrial cancer risk: Results from a population-based case-control study in urban Shanghai. Int J Cancer 2004;108:613-9. [Crossref] [PubMed]
- Win AK, Reece JC, Ryan S. Family history and risk of endometrial cancer: a systematic review and meta-analysis. Obstet Gynecol 2015;125:89-98. [Crossref] [PubMed]
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: An overestimated risk? Eur J Cancer 2017;84:60-8. [Crossref] [PubMed]
- Kralickova M, Losan P, Vetvicka V. Endometriosis and cancer. Womens Health (Lond) 2014;10:591-7. [Crossref] [PubMed]
- Liu SG, Wu XX, Hua T, et al. NLRP3 inflammasome activation by estrogen promotes the progression of human endometrial cancer. Onco Targets Ther 2019;12:6927-36. [Crossref] [PubMed]
- Li H, Mao H, Yu Y, et al. Association between dietary fiber and endometrial cancer: a meta-analysis. Nutr Cancer 2020;72:959-67. [Crossref] [PubMed]
- Guo H, Kong W, Zhang L, et al. Reversal of obesity-driven aggressiveness of endometrial cancer by metformin. Am J Cancer Res 2019;9:2170-93. [PubMed]
- Chaves GV, de Almeida Simao T, Pinto LFR, et al. Overweight and obesity do not determine worst prognosis in endometrioid endometrial carcinoma. Arch Gynecol Obstet 2019;300:1671-7. [Crossref] [PubMed]
- Yang X, Wang J. The Role of Metabolic Syndrome in Endometrial Cancer: A Review. Front Oncol 2019;9:744. [Crossref] [PubMed]
- Abu-Lubad MA, Jarajreh DA, Helaly GF, et al. Human papillomavirus as an independent risk factor of invasive cervical and endometrial carcinomas in Jordan. J Infect Public Health 2020;13:613-8. [Crossref] [PubMed]
- Casarin J, Bogani G, Serati M, et al. Presence of Glandular Cells at the Preoperative Cervical Cytology and Local Recurrence in Endometrial Cancer. Int J Gynecol Pathol 2020;39:522-8. [Crossref] [PubMed]
- Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer 2019;19:510-21. [Crossref] [PubMed]
- Liu H, Wan J, Chu J. Long non-coding RNAs and endometrial cancer. Biomed Pharmacother 2019;119:109396. [Crossref] [PubMed]
- Zhao D, Ren C, Yao Y, et al. Identifying prognostic biomarkers in endometrial carcinoma based on ceRNA network. J Cell Biochem 2020;121:2437-46. [Crossref] [PubMed]
- Liu J, Tan H, Yang W, et al. The voltage-gated sodium channel Nav1.7 associated with endometrial cancer. J Cancer 2019;10:4954-60. [Crossref] [PubMed]
- Bai JDK, Babu S, Roa-Pena L, et al. Keratin 17 is a negative prognostic biomarker in high-grade endometrial carcinomas. Hum Pathol 2019;94:40-50. [Crossref] [PubMed]
- Roa-Pena L, Leiton CV, Babu S, et al. Keratin 17 identifies the most lethal molecular subtype of pancreatic cancer. Sci Rep 2019;9:11239. [Crossref] [PubMed]
- Qian D, Lu Z, Xu Q, et al. Galectin-1-driven upregulation of SDF-1 in pancreatic stellate cells promotes pancreatic cancer metastasis. Cancer Lett 2017;397:43-51. [Crossref] [PubMed]
- Sun XF, Dai SY. The Significance of Galectin-1 and Galectin-9 Expression in Endometrial Carcinoma. Gynecol Obstet Invest 2020;85:34-40. [Crossref] [PubMed]
- Cheng SC, Chen K, Chiu CY, et al. Metabolomic biomarkers in cervicovaginal fluid for detecting endometrial cancer through nuclear magnetic resonance spectroscopy. Metabolomics 2019;15:146. [Crossref] [PubMed]
- Njoku K, Chiasserini D, Whetton AD, et al. Proteomic Biomarkers for the Detection of Endometrial Cancer. Cancers (Basel) 2019; [Crossref] [PubMed]
- Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol 2016;27:16-41. [Crossref] [PubMed]
- Cancer Genome Atlas Research N. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67-73. [Crossref] [PubMed]
- Rizzuto I, Nicholson R, MacNab WS, et al. Risk factors and sonographic endometrial thickness as predictors of tumour stage and histological subtype of endometrial cancer. Gynecol Oncol Rep 2019;30:100491. [Crossref] [PubMed]
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol 2008;109:11-8. [Crossref] [PubMed]
- Ferriss JS, Fader AN. Enough already: Is this the end of comprehensive lymphadenectomy in endometrial cancer or are further trials needed? Gynecol Oncol 2019;155:175-6. [Crossref] [PubMed]
- Nayot D, Kwon JS, Carey MS, et al. Does preoperative positron emission tomography with computed tomography predict nodal status in endometrial cancer? A pilot study. Curr Oncol 2008;15:123-5. [PubMed]
- Gee MS, Atri M, Bandos AI, et al. Identification of Distant Metastatic Disease in Uterine Cervical and Endometrial Cancers with FDG PET/CT: Analysis from the ACRIN 6671/GOG 0233 Multicenter Trial. Radiology 2018;287:176-84. [Crossref] [PubMed]
- Erdogan M, Erdemoglu E, Evrimler S, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis assessed by 18F-FDG PET/CT in endometrial cancer. Nucl Med Commun 2019;40:1099-104. [Crossref] [PubMed]
- Cakir C, Kilic IC, Yuksel D, et al. Does tumor size have prognostic value in patients undergoing lymphadenectomy in endometrioid-type endometrial cancer confined to the uterine corpus? Turk J Med Sci 2019;49:1403-10. [Crossref] [PubMed]
- Li M, Wu S, Xie Y, et al. Cervical invasion, lymphovascular space invasion, and ovarian metastasis as predictors of lymph node metastasis and poor outcome on stages I to III endometrial cancers: a single-center retrospective study. World J Surg Oncol 2019;17:193. [Crossref] [PubMed]
- Donkers H, Bekkers R, Massuger L, et al. Socioeconomic deprivation and survival in endometrial cancer: The effect of BMI. Gynecol Oncol 2020;156:178-84. [Crossref] [PubMed]
- Clarke MA, Long BJ, Sherman ME, et al. A prospective clinical cohort study of women at increased risk for endometrial cancer. Gynecol Oncol 2020;156:169-77. [Crossref] [PubMed]
- Rei M, Rodrigues I, Condeco P, et al. Endometrial cancer: Preoperative versus intraoperative staging. J Gynecol Obstet Hum Reprod 2019; [Crossref] [PubMed]
- Cignini P, Vitale SG, Lagana AS, et al. Preoperative work-up for definition of lymph node risk involvement in early stage endometrial cancer: 5-year follow-up. Updates Surg 2017;69:75-82. [Crossref] [PubMed]
- Bi Q, Chen Y, Wu K, et al. The Diagnostic Value of MRI for Preoperative Staging in Patients with Endometrial Cancer: A Meta-Analysis. Acad Radiol 2020;27:960-8. [Crossref] [PubMed]
- Du J, Zhang F, Zhang L, et al. MicroRNA-103 regulates the progression in endometrial carcinoma through ZO-1. Int J Immunopathol Pharmacol 2019;33:2058738419872621. [PubMed]
- Li S, Zhou J, Wang Z, et al. Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR-196a-5p. Biomed Pharmacother 2018;104:451-7. [Crossref] [PubMed]
- Ghafouri-Fard S, Taheri M. Nuclear Enriched Abundant Transcript 1 (NEAT1): A long non-coding RNA with diverse functions in tumorigenesis. Biomed Pharmacother 2019;111:51-9. [Crossref] [PubMed]
- Wang W, Ge L, Xu XJ, et al. LncRNA NEAT1 promotes endometrial cancer cell proliferation, migration and invasion by regulating the miR-144-3p/EZH2 axis. Radiol Oncol 2019;53:434-42. [Crossref] [PubMed]
- Stasenko M, Tunnage I, Ashley CW, et al. Clinical outcomes of patients with POLE mutated endometrioid endometrial cancer. Gynecol Oncol 2020;156:194-202. [Crossref] [PubMed]
- Guo J, Zhang Y, Qian H, et al. The clinical characteristics and prognosis of endometrial carcinomas that occur after breast cancer: does hormone receptor status of breast cancer matter? Arch Gynecol Obstet 2019;300:1399-404. [Crossref] [PubMed]
- Roh JW, Choi JE, Han HD, et al. Clinical and biological significance of EZH2 expression in endometrial cancer. Cancer Biol Ther 2020;21:147-56. [Crossref] [PubMed]
- Lei S, Zhang Q, Yin F, et al. Expression and clinical significance of KLK5-8 in endometrial cancer. Am J Transl Res 2019;11:4180-91. [PubMed]
- Harris KL, Maurer KA, Jarboe E, et al. LVSI positive and NX in early endometrial cancer: Surgical restaging (and no further treatment if N0), or adjuvant ERT? Gynecol Oncol 2020;156:243-50. [Crossref] [PubMed]
- Miller MD, Devor EJ. Integration of Clinical and Molecular Features into Prediction Models for Outcomes in Endometrial Cancer. Clin Obstet Gynecol 2020;63:40-7. [Crossref] [PubMed]
- Kilic C, Cakir C, Yuksel D, et al. High-grade uterine corpus-confined endometrial cancer with lymphadenectomy: does adjuvant therapy improve survival? Turk J Obstet Gynecol 2019;16:180-6. [Crossref] [PubMed]
- Pal N, Broaddus RR, Urbauer DL, et al. Treatment of Low-Risk Endometrial Cancer and Complex Atypical Hyperplasia With the Levonorgestrel-Releasing Intrauterine Device. Obstet Gynecol 2018;131:109-16. [Crossref] [PubMed]
- DeLair DF, Burke KA, Selenica P, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol 2017;243:230-41. [Crossref] [PubMed]
- Neri M, Peiretti M, Melis GB, et al. Systemic therapy for the treatment of endometrial cancer. Expert Opin Pharmacother 2019;20:2019-32. [Crossref] [PubMed]
- Lheureux S, Oza AM. Endometrial cancer-targeted therapies myth or reality? Review of current targeted treatments. Eur J Cancer 2016;59:99-108. [Crossref] [PubMed]
- Vitale SG, Capriglione S, Zito G, et al. Management of endometrial, ovarian and cervical cancer in the elderly: current approach to a challenging condition. Arch Gynecol Obstet 2019;299:299-315. [Crossref] [PubMed]
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer 2006;95:266-71. [Crossref] [PubMed]
- Boothe D, Orton A, Kim J, et al. Does Early Chemotherapy Improve Survival in Advanced Endometrial Cancer? Am J Clin Oncol 2019;42:813-7. [Crossref] [PubMed]
- Franchi M, Garzon S, Zorzato PC, et al. PET-CT scan in the preoperative workup of early stage intermediate- and high-risk endometrial cancer. Minim Invasive Ther Allied Technol 2020;29:232-9. [Crossref] [PubMed]
- Yanarates A, Budak E. Prognostic role of PET/CT in endometrial cancer. Ginekol Pol 2019;90:491-5. [Crossref] [PubMed]
- Giannone G, Attademo L, Scotto G, et al. Endometrial Cancer Stem Cells: Role, Characterization and Therapeutic Implications. Cancers (Basel) 2019;11:1820. [Crossref] [PubMed]
- Li HL, Sun JJ, Ma H, et al. MicroRNA-23a inhibits endometrial cancer cell development by targeting SIX1. Oncol Lett 2019;18:3792-802. [Crossref] [PubMed]
- Roncolato F, Lindemann K, Willson ML, et al. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst Rev 2019;10:CD012160. [Crossref] [PubMed]
- Lengyel CG, Altuna SC, Habeeb BS, et al. The Potential of PI3K/AKT/mTOR Signaling as a Druggable Target for Endometrial and Ovarian Carcinomas. Curr Drug Targets 2020;21:946-61. [Crossref] [PubMed]
- Kong X, Li M, Shao K, et al. Progesterone induces cell apoptosis via the CACNA2D3/Ca2+/p38 MAPK pathway in endometrial cancer. Oncol Rep 2020;43:121-32. [PubMed]
- Zhao Y, Yang Y, Trovik J, et al. Novel PCDH10-Wnt-MALAT1 regulatory axis in endometrioid endometrial adenocarcinoma. Hong Kong Med J 2019;25:17-22. [PubMed]
- Sperling CD, Verdoodt F, Kjaer Hansen M, et al. Statin use and mortality among endometrial cancer patients: a Danish nationwide cohort study. Int J Cancer 2018;143:2668-76. [Crossref] [PubMed]
- Segev Y, Gemer O, Helpman L, et al. An Israeli Gynecologic Oncology Group study of statin use and endometrial cancer prognosis. Int J Gynaecol Obstet 2020;148:79-86. [Crossref] [PubMed]
- Yokoi E, Mabuchi S, Komura N, et al. The role of myeloid-derived suppressor cells in endometrial cancer displaying systemic inflammatory response: clinical and preclinical investigations. Oncoimmunology 2019;8:e1662708. [Crossref] [PubMed]
- McConechy MK, Talhouk A, Li-Chang HH, et al. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol 2015;137:306-10. [Crossref] [PubMed]
- Emons G, Gorchev G, Harter P, et al. Efficacy and safety of AEZS-108 (LHRH agonist linked to doxorubicin) in women with advanced or recurrent endometrial cancer expressing LHRH receptors: a multicenter phase 2 trial (AGO-GYN5). Int J Gynecol Cancer 2014;24:260-5. [Crossref] [PubMed]
- McAlpine JN, Gilks CB. Precision medicine in endometrial cancer. Gynecol Oncol 2019;154:451-3. [Crossref] [PubMed]


