
Clinical and prognostic significance of preoperative lymphocyte-monocyte ratio, neutrophil-lymphocyte ratio and neutrophil-monocyte ratio on esophageal squamous cell carcinoma patients
Introduction
It’s well known that esophageal cancer is one of the most malignant cancer all over the world and only 10–40% esophageal cancer patients will survive for more than 5 years postoperatively (1,2). Meanwhile, China is a high incidence area for esophageal squamous cell carcinoma (ESCC) with the morbidity of 90% (3,4). The 5-year overall survival rate of ESCC patients in stage IIA–III treated by surgical resection alone is from 20.6% to 34.0% (5,6), which is almost the same as the patients administrated with multimodality therapies including surgery, chemotherapy and radiotherapy (7). TNM stage and tumor differentiation were demonstrated as the independent prognostic factor of esophageal cancer in previous reports (8), however, the heterogeneity of prognosis also exists in patients with same stage. Some investigators showed that cancer-related inflammation leads to worse prognosis, and the inflammatory biomarkers play an important role (9). At the same time, the neutrophil-lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and lymphocyte-monocyte ratio (LMR) have been reported to be the independent prognostic factor correlated to breast, gastric and lung cancer (10-12) previously, however, few studies have showed the prognostic role of the inflammatory biomarkers in ESCC, especially the role of neutrophil-monocyte ratio (NMR). What’s more, the methods of optimal cut-off value determination in published esophageal cancer studies were various, some were empirical, therefore, we took the more practical and precise method to determine the optimal cut-off value of LMR, NLR and NMR in order to evaluate whether preoperative LMR, NLR and NMR plays a key role in survival of ESCC patients. We present this article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-2777).
Methods
Patients
A total of 1,883 patients with esophageal cancer who had accepted the radical esophagectomy at the Department of Thoracic Surgery, West China Hospital of Sichuan University from May 2005 to May 2015 were retrospectively reviewed. The exclusion criteria as follows: (I) patients who were lost to follow-up; (II) pathologically confirmed other types of thoracic esophageal cancer except for ESCC; (III) palliative surgery and R1 or R2 resection; (IV) patients with the total removed lymph nodes less than 10 (13); (V) patients who had accepted the preoperative or postoperative neoadjuvant chemotherapy or radiotherapy; (VI) tumor located less than 20cm from incisors; (VII) patients were accompanied with other malignant tumors. The study was approved by the human participants committee of West China Hospital of Sichuan University (the ethical number: 2005-126), and all patients were informed the risk of the operation. The use of their resected specimens and the written consents were obtained preoperatively. Among them, the median follow-up time is 28.77 months with the range from 1.60 to 167.90 months.
Blood sample analysis
Blood samples of hospitalized patients were adopted within 1 week before the surgery, anticoagulated by EDTA-K2 and examined by CELL DYN 1700 automatic hematologic analyzer (Abbott, USA) for blood routine. The complete blood cell (CBC) counts were extracted from the patents’ medical records retrospectively and only the absolute counts of lymphocyte, monocyte and neutrophil were obtained from CBC data.
LMR, NLR and NMR calculation
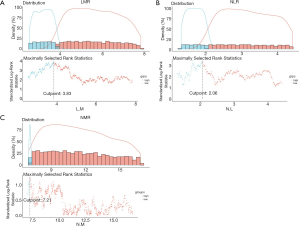
The LMR was calculated by dividing the absolute lymphocyte count by absolute monocyte count. The NLR was calculated by dividing the number of absolute neutrophils by the number of absolute lymphocytes. Similarly, absolute neutrophil counts divided by the absolute monocyte counts is NMR. “Survminer” package in R® Version 3.4.0 (http://www.r-project.org/) was applied to determine the optimal cut-off point of LMR, NLR and NMR, which can provide a value of a cut-off point that correspond to the most significant relation with survival. The optimal cut-off point of LMR, NLR and NMR is 3.83, 2.06 and 7.21, respectively (Figure 1A,B,C).
Follow up
In our study, patients should be followed up every 3 months for the first and second year, every 6 months for the third to fifth year after the treatment, and finally the follow up will be transformed as every year after the fifth year. Blood routine, gastroscopy, chest CT, neck and abdominal ultrasound, when necessary, according to the patient’s symptoms and physical examination. The tumor status (including tumor metastasis and recurrence), patients’ status (including survive and death) as well as the patients who were lost to follow up were all documented not only through outpatient follow up but also through telephone follow up and letter follow up.
Statistical analysis
The clinicopathologic features for each category (LMR, NMR and NLR) were showed in Table 1. In each category, the included patients were divided into two separated groups with regard to the optimal cut-off point. The logistic regression analysis was performed to determine the independent factors related to each category. The overall survival of each category was exhibited from the Kaplan-Meier curves and the log-rank test was used to determine the statistical significance. Multivariate survival analysis was figured out through the Cox proportional hazard regression model. The statistical significance was regarded as the probability value <0.05, and all the statistical analysis were conduct by IBM® SPSS® Statistics Version 21.0.
Table 1
| Characteristics | LMR | NMR | NLR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <3.83 [620] | ≥3.83 [1,263] | P value | <7.21 [221] | ≥7.21 [1,662] | P value | <2.06 [794] | ≥2.06 [1,089] | P value | |||
| Gender | <0.001 | 0.196 | <0.001 | ||||||||
| Male | 566 | 984 | 187 | 1,363 | 599 | 951 | |||||
| Female | 149 | 279 | 34 | 299 | 195 | 138 | |||||
| Age | 0.148 | 0.163 | 0.177 | ||||||||
| <55 | 149 | 275 | 56 | 368 | 170 | 254 | |||||
| ≥55 | 471 | 988 | 165 | 1,294 | 624 | 835 | |||||
| Surgical approach | 0.651 | 0.106 | 0.288 | ||||||||
| Open | 593 | 1,196 | 210 | 1,579 | 747 | 1,042 | |||||
| Minimally | 8 | 18 | 6 | 20 | 13 | 13 | |||||
| Hybrid | 19 | 49 | 5 | 63 | 34 | 34 | |||||
| T stage | <0.001 | 0.577 | <0.001 | ||||||||
| T1 | 69 | 236 | 29 | 276 | 165 | 140 | |||||
| T2 | 83 | 241 | 40 | 284 | 163 | 161 | |||||
| T3 | 328 | 599 | 110 | 817 | 352 | 575 | |||||
| T4 | 140 | 187 | 42 | 285 | 114 | 213 | |||||
| N stage | 0.016 | 0.905 | 0.105 | ||||||||
| N0 | 311 | 703 | 115 | 899 | 447 | 567 | |||||
| N1 | 167 | 327 | 58 | 436 | 199 | 295 | |||||
| N2 | 109 | 173 | 36 | 246 | 101 | 181 | |||||
| N3 | 33 | 60 | 12 | 81 | 47 | 46 | |||||
| M stage | 0.014 | 0.577 | 0.045 | ||||||||
| M0 | 610 | 1,257 | 219 | 1,648 | 791 | 1076 | |||||
| M1 | 10 | 6 | 2 | 14 | 3 | 13 | |||||
| Differentiation | 0.040 | 0.127 | 0.520 | ||||||||
| High | 86 | 190 | 23 | 253 | 125 | 151 | |||||
| Moderate | 360 | 786 | 137 | 1,009 | 476 | 670 | |||||
| Low | 174 | 287 | 61 | 400 | 193 | 268 | |||||
| Location | 0.014 | 0.879 | 0.520 | ||||||||
| Upper | 61 | 123 | 20 | 164 | 73 | 111 | |||||
| Middle | 340 | 775 | 134 | 981 | 485 | 630 | |||||
| Lower | 219 | 365 | 67 | 517 | 236 | 348 | |||||
| Vascular invasion | 0.477 | 0.523 | 0.413 | ||||||||
| No | 590 | 1,204 | 211 | 1,583 | 758 | 1,036 | |||||
| Yes | 30 | 59 | 10 | 79 | 36 | 53 | |||||
| TNM stage | <0.001 | 0.737 | <0.001 | ||||||||
| I | 64 | 212 | 27 | 249 | 143 | 133 | |||||
| II | 221 | 494 | 88 | 627 | 316 | 399 | |||||
| III | 325 | 551 | 104 | 772 | 332 | 544 | |||||
| IV | 10 | 6 | 2 | 14 | 3 | 13 | |||||
| Recurrence | 0.011 | 0.109 | 0.004 | ||||||||
| No | 442 | 964 | 157 | 1,249 | 618 | 788 | |||||
| Yes | 178 | 299 | 64 | 413 | 176 | 301 | |||||
ESCC, esophageal squamous cell carcinoma; LMR, lymphocyte-monocyte ratio; NLR, neutrophil-lymphocyte ratio; NMR, neutrophil-monocyte ratio; T stage, tumor stage; N stage, node stage; M stage, metastasis stage; TNM stage, tumor node metastasis stage.
Results
All patients
A total of 1,883 ESCC patients who met the inclusion criteria were finally enrolled in our study. The median (range) age of the enrolled patients was 60 (range, 20–92) years while the median survival time was 17.20 (range, 0.03–77.80) months. The clinicopathological features of all enrolled patients are presented in Table 1.
Clinical correlation of LMR, NMR and NLR
LMR
All patients were divided into two groups (LMR<3.83 and LMR ≥3.83) according to the cut-off point of LMR, in which 620 patients was in LMR<3.83 group while 1263 patients were classified into LMR ≥3.83 group. The clinicopathological characters of patients in LMR category were exhibited in Table 1. Gender (P<0.001) and T stage (P<0.001) showed significant differences among all the enrolled patients in LMR category through the univariate analysis of logistic regression. Meanwhile, the results of multivariate analysis in logistic regression showed that Gender (P<0.001 ; OR =2.735, 95% CI: 1.999, 3.743) and T stage (P<0.001; OR =0.721, 95% CI: 0.605, 0.858) were the independent factors correlated to enrolled ESCC patients in LMR category as well (Table 2).
Table 2
| Variable | LMR | NMR | NLR | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analyses | Multivariate analyses | Univariate analyses | Multivariate analyses | Univariate analyses | Multivariate analyses | ||||||||||||
| P | OR, 95% CI | P | OR, 95% CI | P | OR, 95% CI | P | OR, 95% CI | P | OR, 95% CI | P | OR, 95% CI | ||||||
| Gender | 0.000 | 2.972 (2.181, 4.049) | 0.000 | 2.735 (1.999, 3.743) | 0.341 | 1.207 (0.820, 1.775) | – | – | 0.000 | 0.446 (0.350, 0.567) | 0.000 | 0.478 (0.374, 0.610) | |||||
| Age | 0.270 | 1.137 (0.905, 1.427) | – | – | 0.286 | 1.193 (0.863, 1.651) | – | – | 0.326 | 0.896 (0.719, 1.116) | – | – | |||||
| Surgical approach | 0.355 | 1.129 (0.873, 1.462) | – | – | 0.579 | 1.117 (0.756, 1.649) | – | – | 0.129 | 0.835 (0.662, 1.054) | – | – | |||||
| T stage | 0.000 | 0.715 (0.642, 0.796) | 0.000 | 0.721 (0.605, 0.858) | 0.248 | 0.915 (0.786, 1.064) | – | – | 0.000 | 1.353 (1.227, 1.493) | 0.000 | 1.333 (1.147, 1.549) | |||||
| N stage | 0.017 | 0.879 (0.791, 0.977) | 0.649 | 0.965 (0.828, 1.125) | 0.468 | 0.945 (0.810, 1.102) | – | – | 0.257 | 1.061 (0.958, 1.176) | – | – | |||||
| M stage | 0.017 | 0.291 (0.105, 0.805) | 0.054 | 0.337 (0.111, 1.019) | 0.924 | 0.930 (0.210, 4.120) | – | – | 0.071 | 3.186 (0.905, 1.216) | – | – | |||||
| Differentiation | 0.032 | 0.843 (0.721, 0.985) | 0.177 | 0.892 (0.756, 1.053) | 0.059 | 0.803 (0.638, 1.009) | – | – | 0.450 | 1.059 (0.913, 1.228) | – | – | |||||
| Location | 0.032 | 0.839 (0.715, 0.985) | 0.406 | 0.932 (0.790, 1.100) | 0.995 | 0.999 (0.792, 1.261) | – | – | 0.661 | 1.035 (0.889, 1.204) | – | – | |||||
| Vascular invasion | 0.872 | 0.964 (0.614, 1.512) | – | – | 0.881 | 1.053 (0.537, 2.065) | – | – | 0.737 | 1.077 (0.698, 1.662) | – | – | |||||
| TNM stage | 0.000 | 0.712 (0.622, 0.816) | 0.403 | 1.130 (0.849, 1.503) | 0.519 | 0.938 (0.773, 1.139) | – | – | 0.000 | 1.342 (1.184, 1.522) | 0.637 | 0.954 (0.786, 1.159) | |||||
| Recurrence | 0.018 | 0.770 (0.620, 0.957) | 0.186 | 0.858 (0.684, 1.077) | 0.188 | 0.811 (0.594, 1.107) | – | – | 0.007 | 1.341 (1.083, 1.661) | 0.062 | 1.234 (0.990, 1.539) | |||||
ESCC, esophageal squamous cell carcinoma; LMR, lymphocyte-monocyte ratio; NLR, neutrophil-lymphocyte ratio; NMR, neutrophil-monocyte ratio; T stage, tumor stage; N stage, node stage; M stage, metastasis stage; TNM stage, tumor node metastasis stage; OR, odds ratio; 95% CI, 95% confidence interval.
NMR
According to the cut-off point of 7.21 in NMR, all patients were divided into two groups as well with 221 patients in NMR<7.21 group and 1,662 patients in NMR≥7.21 group. The clinicopathological features of patients in NMR category were showed in Table 1 and none of the clinical features were significantly associated with NMR (Table 2).
NLR
Seven hundred ninety four patients were classified into the group of NLR <2.06 and the Table 1 also listed the clinicopathological characters of patients in NLR category. From the univariate analysis of logistic regression, Gender (P<0.001) and T stage (P<0.001) showed significant differences among all the enrolled patients in NLR category, at the same time, Gender (P<0.001; OR =0.478, 95% CI: 0.374, 0.610) and T stage (P<0.001; OR=1.333, 95% CI: 1.147, 1.549) were demonstrated as the independent factors associated with NLR through the multivariate analysis of logistic regression (Table 2).
Prognostic value of LMR, NMR and NLR
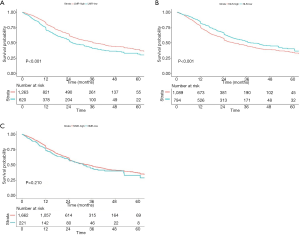
In LMR category, the Kaplan-Meier curves showed that patients with low LMR had a worse overall survival (OS) (P<0.001, Figure 2A) when compared with high LMR group. Nevertheless, patients with low NLR had a better 5-year OS when compared with low NLR group (P<0.001, Figure 2B). No significant prognosis was found between the high NMR and low NMR group (P=0.210, Figure 2C), while the 5-year OS rate of patients with high NMR tended to be higher than that of the patients with low NMR.
In the univariate analysis of Cox proportional hazard regression, we found the T stage (P<0.001), N stage (P<0.001), vascular invasion (P=0.017), TNM stage (P<0.001), recurrence (P<0.001), LMR (P<0.001) and NLR (P<0.001) were significantly associated with 5-year OS (Table 3), while no significant difference with 5-year OS was found in NMR (P=0.405). Finally, LMR (P=0.018; HR =0.786, 95% CI: 0.645, 0.959), NLR (P=0.028; HR =1.247, 95% CI: 1.024, 1.519) as well as T stage (P<0.001; HR =1.464, 95% CI: 1.321, 1.622), N stage (P<0.001; HR =1.206, 95% CI: 1.094, 1.330), vascular invasion (P=0.018; HR =1.552, 95% CI: 1.079, 2.234), TNM stage (P<0.001; HR =1.878, 95% CI: 1.644, 2.145) and Recurrence (P<0.001; HR =3.212, 95% CI: 2.676, 3.855) were drawn as the independent prognostic factors for ESCC patients from the multivariate analysis of Cox proportional hazard regression.
Table 3
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | ||
| Gender | 0.019 | 0.755, (0.597, 0.955) | 0.221 | 0.860 (0.675, 1.095) | |
| Age | 0.024 | 1.311 (1.037, 1.658) | 0.082 | 1.236 (0.973, 1.568) | |
| Surgical approach | 0.840 | 0.977 (0.780, 1.224) | |||
| Open | Ref | ||||
| Minimally | 0.507 | 1.250 (0.646, 2.418) | |||
| Hybrid | 0.684 | 0.904 (0.558, 1.467) | |||
| T stage | 0.000 | 1.681 (1.529, 1.849) | 0.000 | 1.464 (1.321, 1.622) | |
| T1 | Ref | Ref | |||
| T2 | 0.000 | 1.998 (1.386, 2.880) | 0.006 | 1.685 (1.165, 2.436) | |
| T3 | 0.000 | 3.323 (2.429, 4.547) | 0.000 | 2.347 (1.694, 3.252) | |
| T4 | 0.000 | 5.073 (3.632, 7.084) | 0.000 | 3.257 (2.289, 4.635) | |
| N stage | 0.000 | 1.529 (1.458, 1.738) | 0.000 | 1.206 (1.094, 1.330) | |
| N0 | Ref | Ref | |||
| N1 | 0.000 | 2.017 (1.645, 2.473) | 0.004 | 1.362 (1.101, 1.685) | |
| N2 | 0.000 | 2.756 (2.183, 3.479) | 0.000 | 1.571 (1.228, 2.009) | |
| N3 | 0.000 | 3.356 (2.315, 4.865) | 0.032 | 1.531 (1.037, 2.262) | |
| M stage | 0.003 | 3.451 (1.537, 7.751) | 0.669 | 1.196 (0.526, 2.721) | |
| Differentiation | 0.000 | 1.285 (1.122, 1.471) | 0.422 | 1.063 (0.916, 1.232) | |
| High | Ref | Ref | |||
| Moderate | 0.011 | 1.408 (1.083, 1.830) | 0.782 | 0.968 (0.722, 1.215) | |
| Low | 0.000 | 1.706 (1.279, 2.277) | 0.212 | 1.170 (0.914, 1.499) | |
| Location | 0.874 | 0.988 (0.852, 1.145) | |||
| Upper | Ref | ||||
| Middle | 0.573 | 0.913 (0.666, 1.252) | |||
| Lower | 0.680 | 0.932 (0.669, 1.300) | |||
| Vascular invasion | 0.017 | 1.548 (1.082, 2.214) | 0.018 | 1.552 (1.079, 2.234) | |
| TNM stage | 0.000 | 2.219 (1.944, 2.532) | 0.000 | 1.878 (1.644, 2.145) | |
| I | Ref | Ref | |||
| II | 0.000 | 1.827 (1.318, 2.533) | 0.002 | 1.692 (1.220, 2.347) | |
| III | 0.000 | 4.343 (3.183, 5.926) | 0.000 | 3.343 (2.440, 4.580) | |
| IV | 0.000 | 11.909 (5.588, 25.380) | 0.000 | 5.910 (2.749, 12.707) | |
| Recurrence | 0.000 | 3.919 (3.287, 4.672) | 0.000 | 3.212 (2.676, 3.855) | |
| LMR | 0.000 | 0.709 (0.549, 0.846) | 0.018 | 0.786 (0.645, 0.959) | |
| NMR | 0.405 | 0.897 (0.696, 1.158) | |||
| NLR | 0.000 | 1.382 (1.159, 1.649) | 0.028 | 1.247 (1.024, 1.519) | |
ESCC, esophageal squamous cell carcinoma; LMR, lymphocyte-monocyte ratio; NLR, neutrophil-lymphocyte ratio; NMR, neutrophil-monocyte ratio; T stage, tumor stage; N stage, node stage; M stage, metastasis stage; TNM stage, tumor node metastasis stage; HR, hazard ratio; 95% CI, 95% confidence interval; Ref, reference.
The prognostic relationship among LMR, NLR and ESCC
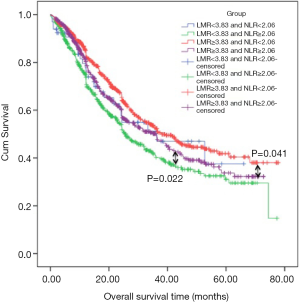
Now that there’s no significant difference of survival in patients of NMR category meanwhile, it was not demonstrated as the independent prognostic factor for ESCC patients, therefore, NMR was excluded from the research of the prognostic relationship between the inflammatory factor and ESCC. We integrated the role of LMR and NLR in prognosis of ESCC patients and the enrolled patients were divided into four groups according to their cut-off points: LMR <3.83 and NLR <2.06 group, LMR <3.83 and NLR ≥2.06 group, LMR ≥3.83 and NLR <2.06 group as well as LMR ≥3.83 and NLR ≥2.06 group. 66, 554, 728 and 535 patients were in the above groups respectively. The Kaplan-Meier curves showed that patients with both LMR and NLR less or greater than their cut-off points got almost the same survival. Patients in LMR ≥3.83 and NLR <2.06 group had the significant better prognosis when compared with patients in LMR ≥3.83 and NLR ≥2.06 group (P=0.041, Figure 3) and patients in LMR<3.83 and NLR ≥2.06 group (P<0.001). At the same time, patients in LMR <3.83 and NLR ≥2.06 group had a worse overall survival compared with patients in LMR ≥3.83 and NLR ≥2.06 group (P=0.022, Figure 3). No significant prognosis was found among patients in LMR<3.83 and NLR <2.06 group and patients in other groups. In the univariate analysis of Cox proportional hazard regression, the group was significantly associated with OS (P=0.030; HR =0.912, 95% CI: 0.839, 0.991), while, it was not recognized as the independent prognostic factor for ESCC patients through multivariate analysis.
Discussion
To our knowledge, the inflammatory factors such as C-reactive protein (CRP) levels, neutrophil, lymphocyte, monocyte and platelet counts in blood may play the prognostic role on various cancers and several studies have reported that the NLR, LMR and PLR were associated with the prognosis with ESCC (14-16). However, neither were the results of these studies consistent, nor were they conclusive, especially for the results of LMR. And there’s even no study about the role of NMR in prognosis of ESCC but in breast cancer (17). Some of the scholars put neoadjuvant or/and adjuvant into their researches, however, neoadjuvant or/and adjuvant therapy has been confirmed as the independent prognostic factor of ESCC (18,19), meanwhile, chemotherapy agents also have been proved to modulated the immune response of cancer (20), which, to a degree, did reflect the prognostic of the inflammatory factors in ESCC authentically (21-23). Furthermore, the methods for determining optimal cut-off value of these inflammatory ratios in different studies were various, some chose the median value of the ratio (22,24), while others picked the Receiver operating characteristics (ROC) curve analysis for the optimal cut-off value determination (16,17,23,25). Therefore, we conducted the retrospective study not only for determining the optimal cut-off values of LMR, NLR and NMR by means of R®, but also for systematically finding out the impact of LMR, NLR and NMR on ESCC patients without receiving adjuvant therapy pre-or postoperatively.
In present study, 1,883 ESCC patients were finally enrolled. Both Gender and T stage were demonstrated as the independent factor correlated to LMR and NLR except for NMR. What’s more, more male patients were found to be associated with high NLR (P<0.001; OR =0.478, 95% CI: 0.374, 0.610) and low LMR (P<0.001; OR =2.735, 95% CI: 1.999, 3.743). Meanwhile, the deeper of the depth of tumor invasion (T stage), the higher NLR (P<0.001; OR =1.333, 95% CI: 1.147, 1.549) as well as the lower LMR (P<0.001; OR =0.721, 95% CI: 0.605, 0.858) were detected. Sun et al. (26) reviewed twenty-six studies including 8,586 ESCC patients for analyzing the clinical use of NLR, PLR and LMR, and the results were calculated as same as ours with high NLR was associated with gender (P=0.002; OR =1.58, 95% CI: 1.19, 2.10) and T stage (P<0.001; OR =1.95, 95% CI: 1.46, 2.60) and low LMR was relevant to gender (P=0.049; OR =0.61, 95% CI: 0.37, 1.00) and T stage (P<0.001; OR =0.58, 95% CI: 0.48, 0.69). With the deeper infiltration of esophageal cancer, the symptoms of esophageal obstruction will get worse and worse. Aspiration pneumonia, cachexia and electrolyte disorders are considered to be the manifestation of advanced esophageal cancer, especially for aspiration pneumonia. And fevers, weight and malnutrition may all be attributed to tumor-induced inflammation (27). Meanwhile, neutrophils are the main inflammatory cells that participate in the tumor microenvironment and promote tumor proliferation, metastasis and invasion (28-31). The main component of human anti-tumor immunity is lymphocytes. Studies have confirmed that the reduction of lymphocytes in tumor stroma will benefit for tumor proliferation and metastasis, leading to the poor prognosis consequently (32). No clinicopathological characters of ESCC patients were significantly correlated to NMR, and Losada et al. (17) have once analyzed the role of pretreatment NMR in 113 breast cancer patients, and no significant differences among patient characters were found in NMR category as well, which was the same result as we did.
As for the prognosis of LMR, in our study we found the low LMR was correlated to worse 5-year OS in ESCC patients. Actually, the mechanism between them is still unclear. However, the increasing investigation has indicated that the inflammatory factors contribute to tumor development, progression and metastasis, especially for NLR and LMR (33). Lymphocytes were reported to be critical in cell-mediated antitumor immune response (34). The CD8+T cell was infiltrated and activated by CD4+T cell, which induce apoptosis of tumor cells and have cytotoxic activity against cancer cells (35). What’s more, tumor cells may escape from host immune surveillance attribute to the inhibition of lymphocytes cytotoxic response initiated by the tumor-related systemic inflammation. Finally, the tumor cell proliferation and migration were suppressed as well as the micrometastases or residual tumor cell were eliminated (36). Furthermore, it’s reported that the lymphocyte counts were low in some cancers, which contributed to the inadequate immune response, leading to the lower survival subsequently (37).
In terms of monocytes, it’s reported that tumor-associated macrophages (TAMs) were a primary part of the mononuclear leukocyte population in human solid tumors (38), originating form circulating monocytes, which played a central role in tumor angiogenesis, invasion, migration, metastasis and inhibition of autoimmune response towards tumor cells (39,40). Some studies have reported the TAM count had a positive correlation with the peripheral blood macrophage percentage and would lead a worse prognosis in multiple cancers if the TAM presented a high infiltration (41). Therefore, not only can circulating monocytes take the place of TAMs in peripheral blood to reflect tumor burden but also it combined with lymphocytes may be considered as a potential, representative biomarker of host immunity versus tumor microenvironment.
Neutrophils, which proliferate and differentiate in bone marrow, is activated by some inflammatory mediators such as granulocyte and granulocyte-macrophage colony stimulating factors (42). The mature neutrophils in human systemic inflammation have been identified as a unique circulating population of myeloid cells (28). Some researches demonstrated that not only did neutrophils produce the factors, which participant in tumor angiogenesis, invasion, migration and metastasis, but also it is capable of inhibiting T cell responses (28-30). Finally, tumor progression decreases with the suppression of neutrophil infiltration. In our study, elevated NLR was associated with worse 5-year OS in ESCC patients, which confirmed the role of neutrophils in tumor patients on the one hand. On the other hand, several studies got the same results as we did (15,16,21-23). However, no significant prognosis was found between the high NMR and low NMR group nor was NMR found to be the independent prognostic factor of ESCC. The results of Kaplan-Meier analysis of NMR in Losada’s study also showed no significant difference of DFS existed in either all breast cancer patients (P=0.45) or triple-negative breast cancer (TNBC) patients (P=0.09) (17). The reason may come from the following respects: one is the amount of studies correlated to the role of NMR in malignant cancer is too small; another may be explained by discrepancies in study population, treatment modality and pathological type.
Integrating LMR and NLR, we analyzed the prognostic relationship among LMR, NLR and ESCC, in which we found the patients in LMR ≥3.83 and NLR <2.06 group received the best prognosis when compared with that of other three groups and the prognosis of patients in LMR<3.83 and NLR ≥2.06 group was the worst. The difference was statistically significant. To our knowledge, seldom have the studies integrated the effect of LMR and NLR on ESCC patients, and what we have received from the study demonstrated that if the lymphocytes or/and monocytes count take the dominant place in tumor-related systemic inflammation, the ESCC patients will harvest the good prognosis, otherwise, the neutrophils will have adverse effect on patients’ survival. Certainly, the further studies are also warranted to confirm the results.
Also, there’re also some limitations in our study. First, our study is a single-center design, retrospective study and the analytical and selection biases were inevitable. In addition, it is difficult to compare our results with those of other studies, which used different cut-off points. As far as we know, we firstly applied R® to determine the optimal cut-off point for LMR, NLR and NMR, which can provide a value of a cut-off point that correspond to the most significant relation with survival. Therefore, it is unclear which is the best approach for cut-off determination and whether a different cutoff value would serve as a better predictor of tumor recurrence in ESCC. Thirdly, the prognostic significance of LMR, NLR and NMR were mainly focused on ESCC patients owing to the most patients with esophageal cancer in China are squamous cell carcinoma, however, the most esophageal cancer in western countries is adenocarcinoma. Thus, a multicenter collaborative prospective study is required to be further verified in a prospective, large-scale collaborative study.
In conclusion, different from the previous studies, we made used of R® to determine the optimal cut-off values and the preoperative LMR as well as NLR may be recognized as the convenient and inexpensive standard laboratory measurements for predicting the prognosis of ESCC patients who received the curative surgery. Meanwhile, better prognosis is associated with the dominant place of lymphocytes or/and monocytes in tumor-related systemic inflammation, however, neutrophils will counteract on prognosis. This finding may help clinicians to assess the prognosis of ESCC patients after operation. Still the further confirmation is warranted.
Acknowledgments
The authors thank Department of Laboratory of West China Hospital, Sichuan University, China for the substantial work in detecting and analyzing the blood samples.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2777
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2777). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the human participants committee of West China Hospital of Sichuan University (the ethical number: 2005-126), and all patients were informed the risk of the operation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nakagawa S, Kanda T, Kosugi S, et al. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 2004;198:205-11. [Crossref] [PubMed]
- Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562-72. [Crossref] [PubMed]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [Crossref] [PubMed]
- Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer 2005;113:456-63. [Crossref] [PubMed]
- Ping YM, Zhang YD, Du XQ, et al. Surgical treatment experiences of 20 000 cases of esophageal and cardiac cancer. The First International Symposium on Esophageal Cancer and the Seventh National Symposium on Esophageal Cancer in China, 2005:167-71.
- Shao LF, Gao ZR, Xu JL, et al. Surgical treatment of 15707 cases of esophageal cancer and cardiac cancer: a summary of the prevention and treatment of esophageal cancer in Henan province. The First International Symposium on Esophageal Cancer and the Seventh National Symposium on Esophageal Cancer in China, 2005:40-5.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43-66. [Crossref] [PubMed]
- Rice TW, Blackstone EH. Esophageal cancer staging: past, present, and future. Thorac Surg Clin 2013;23:461-9. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Minami S, Ihara S, Kim SH, et al. Lymphocyte to Monocyte Ratio and Modified Glasgow Prognostic Score Predict Prognosis of Lung Adenocarcinoma Without Driver Mutation. World J Oncol 2018;9:13-20. [Crossref] [PubMed]
- Saito H, Kono Y, Murakami Y, et al. Prognostic Significance of the Preoperative Ratio of C-Reactive Protein to Albumin and Neutrophil-Lymphocyte Ratio in Gastric Cancer Patients. World J Surg 2018;42:1819-25. [Crossref] [PubMed]
- Marín Hernández C, Pinero Madrona A, Gil Vazquez PJ, et al. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol 2018;20:476-83. [Crossref] [PubMed]
- Amin MB, Edge S, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017:185-202.
- Hirahara N, Matsubara T, Mizota Y, et al. Prognostic value of preoperative inflammatory response biomarkers in patients with esophageal cancer who undergo a curative thoracoscopic esophagectomy. BMC Surg 2016;16:66. [Crossref] [PubMed]
- Geng Y, Shao Y, Zhu D, et al. Systemic Immune-Inflammation Index Predicts Prognosis of Patients with Esophageal Squamous Cell Carcinoma: A Propensity Score-matched Analysis. Sci Rep 2016;6:39482. [Crossref] [PubMed]
- Han LH, Jia YB, Song QX, et al. Prognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinoma. Asian Pac J Cancer Prev 2015;16:2245-50. [Crossref] [PubMed]
- Losada B, Guerra JA, Malon D, et al. Pretreatment neutrophil/lymphocyte, platelet/lymphocyte, lymphocyte/monocyte, and neutrophil/monocyte ratios and outcome in elderly breast cancer patients. Clin Transl Oncol 2019;21:855-63. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- O’Sullivan K E, Phelan JJ, O’Hanlon C, et al. The role of inflammation in cancer of the esophagus. Expert Rev Gastroenterol Hepatol 2014;8:749-60. [Crossref] [PubMed]
- Ji WH, Jiang YH, Ji YL, et al. Prechemotherapy neutrophil: lymphocyte ratio is superior to the platelet: lymphocyte ratio as a prognostic indicator for locally advanced esophageal squamous cell cancer treated with neoadjuvant chemotherapy. Dis Esophagus 2016;29:403-11. [Crossref] [PubMed]
- Sato Y, Gonda K, Harada M, et al. Increased neutrophil-to-lymphocyte ratio is a novel marker for nutrition, inflammation and chemotherapy outcome in patients with locally advanced and metastatic esophageal squamous cell carcinoma. Biomed Rep 2017;7:79-84. [Crossref] [PubMed]
- Miao C, Zhu S, Pan H, et al. Combined neutrophil-platelet score and hemoglobin level predict survival in esophageal squamous cell carcinoma patients treated with chemoradiotherapy. Oncotarget 2017;8:87971-9. [Crossref] [PubMed]
- Song Q, Wu JZ, Wang S. Low Preoperative Lymphocyte to Monocyte Ratio Serves as a Worse Prognostic Marker in Patients with Esophageal Squamous Cell Carcinoma Undergoing Curative Tumor Resection. J Cancer 2019;10:2057-62. [Crossref] [PubMed]
- Hirahara N, Matsubara T, Kawahara D, et al. Prognostic significance of preoperative inflammatory response biomarkers in patients undergoing curative thoracoscopic esophagectomy for esophageal squamous cell carcinoma. Eur J Surg Oncol 2017;43:493-501. [Crossref] [PubMed]
- Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Manag Res 2018;10:6167-79. [Crossref] [PubMed]
- Moore MM, Chua W, Charles KA, et al. Inflammation and cancer: causes and consequences. Clin Pharmacol Ther 2010;87:504-8. [Crossref] [PubMed]
- Hao S, Andersen M, Yu H. Detection of immune suppressive neutrophils in peripheral blood samples of cancer patients. Am J Blood Res 2013;3:239-45. [PubMed]
- Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res 2001;61:4756-60. [PubMed]
- Brandau S, Moses K, Lang S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: cousins, siblings or twins? Semin Cancer Biol 2013;23:171-82. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- O’Callaghan DS, O’Donnell D, O’Connell F. J Thorac Oncol 2010;5:2024-36. [Crossref] [PubMed]
- Liu JS, Huang Y, Yang X, et al. A nomogram to predict prognostic values of various inflammatory biomarkers in patients with esophageal squamous cell carcinoma. Am J Cancer Res 2015;5:2180-9. [PubMed]
- Man YG, Stojadinovic A, Mason J, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer 2013;4:84-95. [Crossref] [PubMed]
- Zikos TA, Donnenberg AD, Landreneau RJ, et al. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol Immunother 2011;60:819-27. [Crossref] [PubMed]
- Nazir T, Islam A, Omer MO, et al. Lymphocytopenia; induced by vinorelbine, doxorubicin and cisplatin in human cancer patients. Breast Dis 2015;35:1-4. [Crossref] [PubMed]
- Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res 2002;8:2553-62. [PubMed]
- Svennevig JL, Svaar H. Content and distribution of macrophages and lymphocytes in solid malignant human tumours. Int J Cancer 1979;24:754-8. [Crossref] [PubMed]
- Torisu H, Ono M, Kiryu H, et al. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer 2000;85:182-8. [Crossref] [PubMed]
- Liss C, Fekete MJ, Hasina R, et al. Paracrine angiogenic loop between head-and-neck squamous-cell carcinomas and macrophages. Int J Cancer 2001;93:781-5. [Crossref] [PubMed]
- Koh YW, Kang HJ, Park C, et al. The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin's lymphoma: correlation with tumor-associated macrophages. Oncologist 2012;17:871-80. [Crossref] [PubMed]
- Lord BI, Bronchud MH, Owens S, et al. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci U S A 1989;86:9499-503. [Crossref] [PubMed]


