Application of apatinib after multifaceted therapies for metastatic breast cancer
Introduction
Breast cancer has a higher morbidity than the other malignant tumors prevalent in females (1). Ongoing advances made in radiotherapy, chemotherapy, endocrine therapy, targeted therapy, and immunotherapy have contributed to reducing the breast cancer mortality rate each year (2). However, the majority of patients with metastatic breast cancer (MBC) develop tolerance to treatment and eventually fail to respond to most Food and Drug Administration (FDA)-approved breast cancer drugs. There is therefore a need for the development of more effective drugs to be applied in clinical practice.
Anti-angiogenesis has garnered considerable research attention as an avenue of malignant tumor treatment. Vascular endothelial growth factors (VEGFs) and their receptor family contribute substantially to tumor growth (3). The VEGF-A monoclonal antibody bevacizumab, combined with chemotherapy, was found to significantly increase the objective response rate (ORR) and progression-free survival (PFS) of patients with metastatic triple-negative breast cancer as a first choice therapy (4-6). Used as second-line treatment, this combination not only improved PFS, but also tended to enhance overall survival (OS) of patients (7). VEGF receptors include VEGFR-1 (Flt1), VEGFR-2 (KDR), VEGFR-3 (Flt4), platelet-derived growth factor receptors (PDGFRs), and c-KIT (8). Sorafenib and sunitinib are small molecule tyrosine kinase inhibitors (TKIs) that inhibit multiple tyrosine kinases, but some studies have shown that neither has a significant effect on MBC (9,10), despite the fact they are thought to react with multiple VEGFRs simultaneously. These TKIs have, in fact, had some success in treating MBC. Due to their toxicity and limited efficacy, however, they are not widely prescribed.
Apatinib is another small molecule oral TKI that is highly specific for VEGFR-2. This TKI is believed to promote cell proliferation and migration, increase vascular permeability, and play a vital role in tumor progression and vasoformation (11). Apatinib administered alone has been shown to inhibit the growth of cholangiocarcinoma cells in preclinical studies (12), and its combination with chemotherapy could reverse multidrug resistance in multiple cancer cell lines. The combination of apatinib and chemotherapy may also result in cytotoxicity by significantly enhancing the cytotoxicity of ABCB1 or ABCG2 substrate drugs in cells where ABCB1 and ABCG2 (wild type) are overexpressed (13). Clinical research on phase II MBC has shown that apatinib administered alone was effective against both triple-negative and non-triple-negative breast cancer with a median PFS (mPFS) of 3.3 and 4 months, respectively, and a median OS (mOS) of 10.6 months and 10 months, respectively (14,15). However, there is limited clinical research investigating the combination treatment of apatinib and chemotherapy or endocrine therapy.
In the present study, apatinib was applied both as a monotherapy and a combination therapy in MBC patients from our cancer center that had not responded to multifaceted therapy. All 61 patients were retrospectively analyzed to investigate the efficacy and toxicity of apatinib. The findings suggest that apatinib can be a viable treatment option for patients with breast cancer, and our research lays a foundation for further clinical studies into the use of this drug in treatment-resistant MBC patients.
We present the following article in accordance with STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-2588).
Methods
Ethics statement
This research was conducted in line with the Declaration of Helsinki (as revised in 2013) and was ratified by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (IORG No: IORG0003571; No. 2020-S103). All patients provided written informed consent.
Patients
We recruited 61 patients who were treated with apatinib in our department from March 2016 to February 2018 as subjects in our retrospective study. They had all been diagnosed with MBC and had received at least one treatment of anthracycline and taxane chemotherapy. The patients were aged from 30 to 70 years. Hormone receptor-positive (HR+) patients were resistant to endocrine therapy and could not receive other endocrine therapies for financial reasons. Human epidermal growth factor receptor 2 positive (HER-2+) patients had received tastuzumab (Herceptin) treatment and shown drug resistance, but could not receive other anti-HER-2 treatments for financial reasons. These patients were either intolerant to other drugs or rejected the use of other chemotherapeutic agents, and their Eastern Cooperative Oncology Group (ECOG) scores ranged from 0 to 2. Patients elected to receive single-agent or combination therapy with apatinib according to their tolerance and willingness to undergo the treatment.
Exclusion criteria included the following: patients with wounds that did not heal; patients with bone fractures that were traumatic or pathological; patients with urine protein more than 2+ and validated urinary protein/24 h >1.0 g; patients treated by any concomitant antineoplastic therapy; patients with reduced hematologic, hepatic, or renal function; and patients with congestive heart failure or other conditions that increased their risk for toxicity.
Before 2011, ER/PgR negativity was defined as immunohistochemistry (IHC) showing less than 10% positive tumor cells with chromatin. Since 2011, based on the new College of American Pathologists guidelines, ER/PgR negativity has been defined as ER/PgR staining of less than 1%. The status of HER2/Neu-negativity in the present study was defined by an IHC score of 0 to 11 or by chromogenic/fluorescent in situ hybridization (CISH/FISH) in line with the guidelines of the American Society of Clinical Oncology (ASCO).
Treatment
Apatinib dose was modified according to toxicity criteria. Therapy continued until a patient’s disease deteriorated, drug toxicity was unacceptable, or the patient left the study of their own volition. Patient response was evaluated every 2 months. The gradation of adverse events (AEs) was implemented in line with the Common Terminology Criteria for Adverse Events (CTCAE, Version 4.03) issued by the National Cancer Institute in the United States.
Data collection
The baseline clinical characteristics of patients after enrollment were collected and standardized. The clinical benefit rate (CBR) was considered to be the ratio of assessable patients to achieve a complete response (CR), partial response (PR), or stable disease (SD) for more than 4 weeks and was determined using the RECIST standard 1.1 (16).
Statistical analysis
SPSS 19.0 was used to conduct all the statistical analysis in this study. PFS and OS were calculated by using Log-rank analysis. Single-factor analysis of DCR were performed using Chi-square tests, and variables with P value <0.3 in the single-factor Chi-square tests were evaluated in multivariate regression analysis by using Cox proportional hazards regression models. P value <0.05 was considered statistically significant.
Results
Baseline characteristics
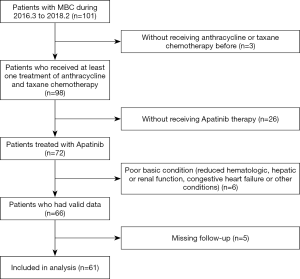
As of February 1, 2018, 61 MBC patients that met our criteria had been observed (Figure 1); the general information of these patients is detailed in Table 1. Of these patients, 19 had triple-negative breast cancer, 26 had HR+ breast cancer, and 13 had HER-2+ breast cancer. Before receiving apatinib treatment, all patients underwent some form of chemotherapy, including neoadjuvant, adjuvant, or post-metastasis chemotherapy, and 31 received three or more cycles of chemotherapy post-metastasis. Patients with HR+ breast cancer had all received endocrine therapy that either failed, could not be tolerated, or could not be afforded. Patients with HER-2+ breast cancer had been treated with Herceptin, and disease progression was observed. Of the 61 patients, 16 had brain metastases, and 11 patients had more than 3 intracranial metastases (Table 1).
Table 1
| Variable | Group | Whole population (n=61), n (%) |
|---|---|---|
| Age (years) | ≥60 | 12 (19.7) |
| <60 | 49 (80.3) | |
| Menopausal state | Post-menopause | 39.3% |
| Pre-menopause | 60.7% | |
| Pathological type | Invasive ductal carcinoma | 58 (95.1) |
| Encephaloid carcinoma | 3 (4.9) | |
| Subgroup | Triple negative | 19 (31.1) |
| HER2 positive | 13 (21.3) | |
| Luminal A | 15 (24.6) | |
| Luminal B | 11 (18.0) | |
| Unknown | 3 (5.0) | |
| Metastatic sites | Lymph node | 24 (39.3) |
| Bone | 32 (52.5) | |
| Lung | 26 (42.6) | |
| Liver | 23 (37.7) | |
| Brain | 16 (26.2) | |
| Chest wall | 8 (13.1) | |
| Pleura | 5 (8.2) | |
| Peritoneum | 3 (4.9) | |
| No. of metastatic >3 | 23 (38.3) | |
| Visceral | 55 (91.7) | |
| Prior chemotherapy regimen | Anthracycline | 43 (70.5) |
| Taxanes | 51 (83.6) | |
| Capecitabine | 43 (70.5) | |
| Vinorelbine | 37 (60.6) | |
| Gemcitabine | 31 (50.8) | |
| Lines of chemotherapy | ≤3 cycles | 30 (49.2) |
| >3 cycles | 31 (50.8) | |
| Combination with Apatinib | None | 9 (14.8) |
| Chemotherapy | 49 (80.3) | |
| Herceptin | 1 (1.6) | |
| Endocrine therapy | 2 (3.3) |
HER2, human epidermal growth factor receptor-2.
The apatinib regimen for a given patient was developed based on tolerability and current condition; 9 patients received monotherapy with apatinib, and 2 patients received apatinib therapy combined with letrozole; a combination of apatinib and taxane drugs were given to 19 patients, while 5 patients received apatinib combined with vinorelbine, and 12 patients received apatinib combined with capecitabine. For 58 patients, the initial dose of apatinib was 250 mg/day. This was reduced to 250 mg every second day in 8 patients after 1 week due to intolerable AEs, with 2 patients discontinuing apatinib therapy due to AEs. Two patients received an initial dose of 500 mg/day, which was reduced to 250 mg/day in one patient after 2 weeks due to intolerable AEs; one patient received an initial dose of apatinib of 250 mg every 2 days.
Safety
The toxicities encountered in our study are listed in Table 2. Due to intolerable AEs, 2 of 61 patients discontinued apatinib treatment. After taking apatinib at a dose of 250 mg/day for 4 days, one patient experienced a small amount of vaginal bleeding that was bright red, intermittent, and persisted approximately 4 days. A reduced dose was recommended, but the patient declined to continue treatment. After 2 weeks of apatinib monotherapy, another patient experienced severe anorexia and fatigue. It was recommended that drug treatment be temporarily suspended to allow time for symptoms to improve, but the patient declined to continue treatment. The chest wall mass in another patient was significantly smaller after 2 weeks of apatinib treatment of 250 mg/day combined with capecitabine, but the remaining metastatic lesions were not evaluated. This patient suffered a gradual onset of gastrointestinal bleeding that mainly manifested as bloody stool. One month after drug treatment ceased, this patient died, with disease progression being considered the cause of death. The remaining 58 patients continued to use apatinib until disease progression occurred. One patient received monotherapy with apatinib at a dose of 500 mg/day until disease progression occurred. For the remainder of the patients, regardless of initial dose and whether apatinib treatment was combined with other drugs or used alone, the dose was reduced to 250 mg/day or 250 mg every second day so it could be tolerated.
Table 2
| Adverse event | Total, n (%) | Grade 1, n (%) | Grade, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|---|---|
| Monotherapy with apatinib | |||||
| Hypertension | 3 (33.3) | 1 (11.1) | 1 (11.1) | 0 (0.0) | 1 (11.1) |
| Hand-foot syndrome | 2 (22.2) | 1 (11.1) | 1 (11.1) | 0 (0.0) | 0 (0.0) |
| Fatigue | 1 (11.1) | 0 (0.0) | 1 (11.1) | 0 (0.0) | 0 (0.0) |
| Mucositis | 2 (22.2) | 2 (22.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bleeding | 2 (22.2) | 1 (11.1) | 1 (11.1) | 0 (0.0) | 0 (0.0) |
| Anorexia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea | 2 (22.2) | 1 (11.1) | 1 (11.1) | 0 (0.0) | 0 (0.0) |
| Proteinuria | 1 (11.1) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Combintherapy with apatinib | |||||
| Hypertension | 27 (44.3) | 1 (1.6) | 12 (19.7) | 13 (21.3) | 2 (3.3) |
| hand-foot syndrome | 22 (36.0) | 6 (9.8) | 8 (13.3) | 8 (13.1) | 0 (0.0) |
| Fatigue | 19 (31.1) | 14 (23.0) | 5 (8.3) | 0 (0.0) | 0 (0.0) |
| Mucositis | 11 (18.0) | 6 (9.8) | 2 (3.3) | 5 (8.2) | 0 (0.0) |
| Bleeding | 10 (16.4) | 6 (9.8) | 3 (4.9) | 0 (0.0) | 1 (1.6) |
| Anorexia | 9 (14.8) | 9 (14.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea | 3 (4.9) | 3 (4.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neutropenia | 29 (47.5) | 3 (4.92) | 16 (26.2) | 9 (14.8) | 1 (1.6) |
| Anemia | 23 (37.7) | 14 (23.0) | 9 (14.8) | 0 (0.0) | 0 (0.0) |
| Thrombocytopenia | 3 (4.9) | 0 (0.0) | 1 (1.6) | 1 (1.6) | 1 (1.6) |
| Transaminase increased | 34 (55.7) | 29 (47.6) | 5 (8.2) | 0 (0.0) | 0 (0.0) |
| Bilirubin increased | 24 (39.3) | 14 (23.0) | 8 (13.1) | 1 (1.6) | 1 (1.6) |
| Proteinuria | 5 (8.2) | 0 (0.0) | 5 (8.2) | 0 (0.0) | 0 (0.0) |
Of the 61 patients, 37 experienced grade 3 AEs, including hypertension, hand-foot syndrome, oral mucositis, and neutropenia. The most frequently occurring grade 2 AEs were neutropenia, hypertension, hand-foot syndrome, and elevated bilirubin. The most frequently occurring grade 1 AEs were elevated transaminase, anemia, elevated bilirubin, fatigue, anorexia, and hand-foot syndrome. Approximately half of the patients experienced hypertension. In addition, 39.3% of the patients experienced hand-foot syndrome, which was self-mitigated by most patients. Oral mucositis was also a common AE, but this was alleviated by gargling with mouthwash containing vitamin B12, dexamethasone, and gentamicin.
Efficacy
Overall efficacy
Of the 61 patients, PR was observed in 14 patients (23.0%), SD in 30 patients (49.2%), and progressive disease (PD) in 17 patients (27.8%). CR was not observed in any patients. The disease control rate (DCR) was 44/61 (72.1%), and the ORR was 14/61 (23.0%). The two patients who declined to continue apatinib treatment showed a reduction in size of their chest wall nodules after 1 week of treatment, but drug efficacy could not be evaluated due to the treatment being discontinued. Of the 44 patients who achieved PR or SD, the PFS was 3 to 6 months and the mPFS was 4.5 months.
Clinical efficacy of apatinib alone and apatinib combined with chemotherapy
Of the 61 patients in the study, 49 were treated with apatinib combined with chemotherapy. Of these patients, 19 (38.8%) received apatinib combined with taxanes, 14 (28.5%) received apatinib combined with capecitabine, 5 (10.2%) received apatinib combined with vinorelbine, 5 (10.2%) received apatinib combined with gemcitabine, 2 (4.1%) received apatinib combined with pemetrexed, 2 (4.1%) received apatinib combined with gimeracil and oteracil potassium capsules, and 2 (4.1%) received apatinib combined with irinotecan. Log-rank analysis showed no difference in PFS (P=0.392) or OS (P=0.412) between patients treated with apatinib alone and those treated with apatinib in combination with chemotherapy.
Clinical efficacies against brain metastases
Sixteen patients had brain metastases (Table 3). Of these patients, the ORR was 8/16 (50%) and the DCR was 13/16 (81.2%). Eleven of these patients had more than three intracranial metastases. Six patients received apatinib therapy combined with radiotherapy with an interval between radiotherapy and medication shorter than 3 months; this was described as the apatinib combined radiotherapy group. Six patients received radiotherapy and apatinib at least half a year apart and four did not receive radiotherapy; this was described as the apatinib group. The intracranial ORR and DCR of the apatinib group were 50% (5/10) and 80% (8/10), respectively, and the intracranial ORR and DCR of the apatinib combined radiotherapy group were 50% (3/6) and 83.3% (5/6), respectively (Table 3).
Table 3
| No. | Brain radiotherapy with apatinib simultaneously | CNS end |
|---|---|---|
| 1 | Yes | PR |
| 6 | No | PR |
| 9 | Yes | SD |
| 10 | No | PR |
| 11 | No | PR |
| 28 | Yes | PD |
| 38 | No | PR |
| 41 | No | SD |
| 42 | Yes | SD |
| 43 | No | PD |
| 46 | No | PR |
| 51 | Yes | PD |
| 53 | No | SD |
| 56 | Yes | PR |
| 58 | Yes | PR |
| 60 | No | SD |
CNS, central nervous system; PR, partial response; SD, stable disease; PD, progressive disease.
Clinical efficacy and expression of P53
Specimens from 11 of 61 patients were subjected to next-generation sequencing (NGS). The TP53 gene of five patients was found to be wild type before and after treatment. Three of these five patients achieved SD and two achieved PR. The TP53 gene of patient no. 11 was mutated before treatment and wild type after treatment; this patient achieved PR and reached pathologic remission. The TP53 gene of five patients were mutated both before and after treatment. Four of these five patients achieved PD and one achieved SD. The ORR and DCR of the mutant P53 group were 0% and 20%, respectively, and the prognosis of these patients was worse than that of the wild-type P53 group (ORR 50% and DCR 100%) (Tables 4,5).
Table 4
| No. | TP53 mutation | End |
|---|---|---|
| 1 | Wild type | PR |
| 10 | Wild type | PR |
| 11 | Mutation: wild type | PR |
| 14 | Wild type | SD |
| 12 | Wild type | SD |
| 2 | Wild type | SD |
| 20 | Mutation | SD |
| 5 | Mutation | PD |
| 29 | Mutation | PD |
| 37 | Mutation | PD |
| 55 | Mutation | PD |
PR, partial response; SD, stable disease; PD, progressive disease.
Table 5
| P53 mutation | Number | ORR, % | DCR, % |
|---|---|---|---|
| P53 (+) | 5 | 0.0 | 20.0 |
| P53 (−) | 6 | 50.0 | 100.0 |
ORR, objective response rate; DCR, disease control rate.
Single-factor and multifactor analysis
The results of Single-factor analyses of DCR showed a clear correlation between hypertension and clinical benefit (P=0.008). Age, menopausal status, histology classification, molecular typing, metastatic site, visceral metastasis, chemotherapy line, hand-foot-skin reaction (HFSR), asthenia, oral mucositis, bleeding, decreased appetite and nausea, and adverse reactions were unrelated to clinical benefit (P>0.05) (Table 6).
Table 6
| Factors | χ2 | P value |
|---|---|---|
| Hypertension | 7.023 | 0.008 |
| HFSR | 0.627 | 0.429 |
| Fatigue | 1.843 | 0.127 |
| Mucositis | 0.295 | 0.587 |
| Bleeding | 0.195 | 0.659 |
| Anorexia | 0.122 | 0.727 |
| Nausea | 0.081 | 0.776 |
| Age | 2.016 | 0.156 |
| Menopausal state | 0.973 | 0.265 |
| Histology classification | 0.627 | 0.429 |
| Molecular typing | 0.189 | 0.664 |
| Metastatic sites | 0.776 | 0.297 |
| Visceral metastasis | 0.889 | 0.276 |
| Lines of chemotherapy | 0.276 | 0.600 |
HFSR, hand-foot-skin reaction.
The results of multivariate regression analysis were shown in Table 7. Hypertension (P=0.044, HR 0.206, 95% CI: 0.044–0.960) had a strong correlation with better DCR. Age, menopausal status, metastatic site, visceral metastasis, and fatigue were not independent prognostic indicators of disease control.
Table 7
| Factors | HR | 95% CI | P value |
|---|---|---|---|
| Age (>50 vs. ≤50 years old) | 3.176 | 0.353–28.684 | 0.303 |
| Menopausal state (post-menopause vs. pre-menopause) | 0.810 | 0.083–7.867 | 0.856 |
| Metastatic sites (>3 vs. ≤3) | 2.414 | 0.532–10.943 | 0.253 |
| Visceral metastasis (yes vs. no) | 0.266 | 0.022–3.226 | 0.298 |
| Hypertension (yes vs. no) | 0.206 | 0.044–0.960 | 0.044 |
| Fatigue (yes vs. no) | 0.323 | 0.051–0.875 | 0.208 |
HR, hazard ratio; CI, confidence interval.
Discussion
As an antiangiogenic small molecule TKI, apatinib has been used to treat a variety of malignant tumors, including gastric cancer (17), esophageal cancer (18), non-small cell lung cancer (19), and breast cancer. A phase II clinical trial of apatinib monotherapy in patients with triple-negative and non-triple-negative metastatic breast tumors has been carried out (14,15). Zhu et al. (20) reported that the combination of apatinib and chemotherapeutic agents may be beneficial in patients with advanced pretreated breast cancer. However, research into the combination of apatinib with chemotherapy or endocrine therapy for MBC treatment remains limited. Apatinib has been reported in preclinical studies to reverse ABCB1/MDR1- and ABCG2 (BCRP/MXR/ABCP)-mediated multidrug tolerance in breast cancer cells. Apatinib blocks the transport functions of these proteins, and this reversal effect is particularly evident after treatment with anthracycline and taxane drugs. Apatinib has also been used with target therapy for cancer stem-like cells and ABCB1-overexpressing leukemia cells to increase the efficacy of chemotherapeutic drugs (21).
In the present study, one patient who had been taking bevacizumab before the administration of apatinib experienced disease progression during treatment. After the patient switched to apatinib combined with albumin paclitaxel, the breast mass rapidly reduced in size. Although the regimen was discontinued due to epistaxis, the tumor reduction allowed the patient to undergo radical mastectomy, after which pathologic remission was achieved. This patient has now completed postoperative radiotherapy that resulted in good local disease control. Although we cannot guarantee this patient will obtain any survival benefit from this treatment, her current quality of life has improved. For example, she has experienced no rupturing of breast masses, which is important in a 36-year-old woman. The functional mechanisms of the antiangiogenic drugs apatinib and bevacizumab are slightly different, and additional studies are needed to more profoundly understand these differences.
All the HER-2+ patients in the present study received trastuzumab therapy, and some received lapatinib and TDM1 therapy. These patients did not receive additional anti-HER-2 therapy for financial reasons. Apatinib was also effective in HER-2+ patients that did not respond to multifaceted therapies; however, determining the optimal time to begin treatment and the manner in which maximum efficacy can be achieved, still requires further exploration.
Of the 16 patients with brain metastases, 6 received apatinib therapy combined with radiotherapy with an interval between radiotherapy and medication of fewer than 3 months; these patients were the apatinib combined radiotherapy group. The single apatinib group comprised 4 patients that had never received radiotherapy and 6 patients that had received radiotherapy and apatinib at least half a year apart. Advanced breast cancer is complex; some patients develop intracranial metastasis quickly and require whole brain radiotherapy. Apatinib is used in patients after multifaceted therapy, so very few patients receive apatinib combined with whole brain radiotherapy. We believe that if the interval between radiotherapy and drug therapy is half a year or longer almost no interaction occurs between these therapies. The intracranial ORR and DCR of the apatinib group were similar to the ORR and DCR of the apatinib combined radiotherapy group. Small molecule TKIs can theoretically cross the blood-brain barrier to treat brain metastases (22,23). However, treatment with small molecule TKIs has not achieved satisfactory clinical efficacy in patients with breast cancer and brain metastases. The use of apatinib for brain metastases has not been frequently reported, and further research is required to further understand its role and clarify its mechanism.
In the present study, 11 of 61 (18%) patients underwent hematologic NGS testing before and after treatment. We found that the TP53 gene of patient no. 11 was mutated before treatment and wild type after treatment. This patient achieved PR and reached pathologic remission. Five patients had a mutated TP53 gene, and four of these five patients achieved PD and one achieved SD. Due to the limited number of patients tested, we could reach no definite conclusions, but the relationship between TP53 gene mutations and breast cancer has long been a concern. In fact, TP53 genes are the most likely gene to be mutated across tumors, yet there is no approved therapy that targets it. A study by Schwaederle et al. showed that there is a clinical correlation between TP53 mutations and better PFS after receiving bevacizumab therapy (24). These results demonstrate that TP53 mutations are independent predictors of the high expression of VEGF-A. Although most studies suggest that TP53 mutations are correlated with the prognosis of various subtypes of breast cancer (25-27), this gene cannot be used as a predictor of therapy sensitivity in breast cancer patients. Sensitivity to apatinib treatment has been shown to be associated with the expression of ABCB1, ABCG2, and VEGFR in tumor tissue, but no presence of predictors in peripheral blood of apatinib sensitive patients has been reported. Based on these 11 patients’ results, we may further study the correlation between TP53 mutations and the efficacy of apatinib to explore whether the change from mutant to wild-type TP53 is a sign of treatment efficacy.
The most common AEs associated with apatinib treatment in our study were hypertension and hand-foot syndrome; approximately half of our patients experienced hypertension. However, our study was based on 61 patients, which is a small number. Therefore, our observations can only serve as a reference for the clinical application of apatinib and as a basis for future studies. In relation to clinical prognosis, adverse reactions during anti-angiogenesis therapy are closely associated with clinical benefit. In a study of apatinib in advanced MBC therapy, patients with hypertension and HFSR all had better PFS, CBR, and OS than those without hypertension (P<0.05) (28). Clinical data from the E2100 study showed that patients with levels 3 and 4 hypertension after bevacizumab treatment had a significant benefit in OS compared to those without hypertension (38.7 vs. 25.3 months, P=0.02) (29). Hypertension of level 2 or greater has been found to be an independent prognostic factor of metastatic gastric cancer (P=0.009) (30). In our study, a Chi-square test was conducted on AEs revealing that hypertension had a significant association with better DCR (P=0.008) during the treatment process, but there was little association between fatigue, oral mucositis bleeding, anorexia, or nausea and DCR. Single-factor and multifactor analyses of DCR showed that hypertension was an independent prognostic factor of DCR (HR 0.206, P=0.044). Therefore, the presence of hypertension after treatment with apatinib is expected to be a marker of better disease control.
Conclusions
Apatinib showed good efficacy and manageable toxicity in patients with MBC who were unresponsive to multifaceted therapy.
Acknowledgments
Funding: The present study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2588
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-19-2588
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2588). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This research was conducted in line with the Declaration of Helsinki (as revised in 2013) and was ratified by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (IORG No: IORG0003571, No. 2020-S103). All patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019;69:438-51. [Crossref] [PubMed]
- Ronca R, Benkheil M, Mitola S, et al. Tumor angiogenesis revisited: Regulators and clinical implications. Med Res Rev 2017;37:1231-74. [Crossref] [PubMed]
- Hamilton E, Kimmick G, Hopkins J, et al. Nab-paclitaxel/bevacizumab/carboplatin chemotherapy in first-line triple negative metastatic breast cancer. Clin Breast Cancer 2013;13:416-20. [Crossref] [PubMed]
- Ferrero JM, Hardy-Bessard AC, Capitain O, et al. Weekly paclitaxel, capecitabine, and bevacizumab with maintenance capecitabine and bevacizumab as first-line therapy for triple-negative, metastatic, or locally advanced breast cancer: Results from the GINECO A-TaXel phase 2 study. Cancer 2016;122:3119-26. [Crossref] [PubMed]
- Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 2011;29:1252-60. [Crossref] [PubMed]
- Brufsky A, Valero V, Tiangco B, et al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat 2012;133:1067-75. [Crossref] [PubMed]
- Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol 2014;41:235-51. [Crossref] [PubMed]
- Moreno-Aspitia A, Morton RF, Hillman DW, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol 2009;27:11-5. [Crossref] [PubMed]
- Barrios CH, Liu MC, Lee SC, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat 2010;121:121-31. [Crossref] [PubMed]
- Geng R, Li J. Apatinib for the treatment of gastric cancer. Expert Opin Pharmacother 2015;16:117-22. [Crossref] [PubMed]
- Peng S, Zhang Y, Peng H, et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by Apatinib. Cancer Lett 2016;373:193-202. [Crossref] [PubMed]
- Mi YJ, Liang YJ, Huang HB, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 2010;70:7981-91. [Crossref] [PubMed]
- Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961-9. [Crossref] [PubMed]
- Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Zhang S. Problematic Analysis and Inadequate Toxicity Data in Phase III Apatinib Trial in Gastric Cancer. J Clin Oncol 2016;34:3821. [Crossref] [PubMed]
- Ter Veer E, Haj Mohammad N, van Valkenhoef G, et al. Second- and third-line systemic therapy in patients with advanced esophagogastric cancer: a systematic review of the literature. Cancer Metastasis Rev 2016;35:439-56. [Crossref] [PubMed]
- Tang J, Li XY, Liang JB, et al. Apatinib Plus Chemotherapy Shows Clinical Activity in Advanced NSCLC: A Retrospective Study. Oncol Res 2019;27:635-41. [Crossref] [PubMed]
- Zhu A, Yuan P, Wang J, et al. Apatinib combined with chemotherapy in patients with previously treated advanced breast cancer: An observational study. Oncol lett 2019;17:4768-78. [PubMed]
- Tong XZ, Wang F, Liang S, et al. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol 2012;83:586-97. [Crossref] [PubMed]
- Duchnowska R, Loibl S, Jassem J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat Rev 2018;67:71-7. [Crossref] [PubMed]
- Nagpal A, Redvers RP, Ling X, et al. Neoadjuvant neratinib promotes ferroptosis and inhibits brain metastasis in a novel syngeneic model of spontaneous HER2 breast cancer metastasis. Breast Cancer Res 2019;21:94. [Crossref] [PubMed]
- Schwaederle M, Lazar V, Validire P, et al. VEGF-A Expression Correlates with TP53 Mutations in Non-Small Cell Lung Cancer: Implications for Antiangiogenesis Therapy. Cancer Res 2015;75:1187-90. [Crossref] [PubMed]
- Bai F, Jin Y, Zhang P, et al. Bioinformatic profiling of prognosis-related genes in the breast cancer immune microenvironment. Aging (Albany NY) 2019;11:9328-47. [Crossref] [PubMed]
- Van Keymeulen A, Lee MY, Ousset M, et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature 2015;525:119-23. [Crossref] [PubMed]
- Skibinski A, Kuperwasser C. The origin of breast tumor heterogeneity. Oncogene 2015;34:5309-16. [Crossref] [PubMed]
- Fan M, Zhang J, Wang Z, et al. Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGF-dependency of advanced breast cancer in anti-angiogenic therapy. Breast Cancer Res Treat 2014;143:141-51. [Crossref] [PubMed]
- Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 2008;26:4672-8. [Crossref] [PubMed]
- Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of Sunitinib activity. Ann Oncol 2007;18:1117. [Crossref] [PubMed]