
Involvement of DNA methyltransferase 1 (DNMT1) and multidrug resistance-associated proteins in 2-methoxyestradiol-induced cytotoxicity in EC109/Taxol cells
Introduction
Esophageal cancer (EC) is the sixth leading cause of cancer mortality and the eighth-most common form of cancer worldwide (1). Further, the incidence of EC is rapidly increasing around the world (2). The 5-year survival rate for esophageal squamous cell carcinoma (ESCC) ranges from 5% to 12.3% in Europe; however, it is more severe in some areas of China (3). In general, chemotherapy has played an important role in the treatment of EC (4-6). Besides, human esophageal carcinoma cells are liable and form multidrug resistance (MDR) after treatment with chemotherapy, which is a primary cause of chemotherapy failure (7,8).
Paclitaxel is an active anticancer agent used to treat a broad spectrum of tumors, including esophageal carcinoma. However, because of the impact of drug resistance, the efficacy of paclitaxel is greatly limited (9,10). Therefore, there is an urgent need to find a drug that induces cytotoxicity in EC109/Taxol cells (11). 2-Methoxyestradiol (2-ME) is a normal physiological metabolite of 17β-estradiol. Due to its low toxicity and broad-spectrum anti-cancer activity, 2-ME is considered a promising anti-cancer drug candidate and is used in phase I and II clinical trials for the treatment of various cancers. EC cells have resistance to paclitaxel treatment (12-17).
EC was faced with multiple drug resistance like other anti-cancer agents. There are a few assumptions about the drug resistance mechanisms of anti-cancer agents. For example, increasing numbers of studies implicate that there is abnormal DNA methyltransferase 1 (DNMT1) in cancer (18). And DNMT1 is one of the key factors in maintaining drug resistance (19). It also has been shown the over-expression of some ATP-binding cassette (ABC) transporters could induce the resistance of cancer and tumor cells, which may finally lead to chemotherapy failure (20). These factors may lead to the failure of EC chemotherapy.
It is hypothesized in this paper that paclitaxel resistance is associated with alterations in the expression of DNMT1 and MDR-associated proteins. In this study, the mechanisms underlying 2-ME-induced cytotoxicity in EC109/Taxol cells are investigated. The results of this study demonstrate that paclitaxel resistance is associated with a decrease in DNMT activity. In addition, this study also found that, in EC109/Taxol cells resistant to paclitaxel (Taxol) treatment, 2-ME decreases DNMT1-mediated paclitaxel resistance by simultaneously reducing the expression of ABC transporters, including phosphoglycoprotein (P-gp), breast cancer resistance protein (BCRP), and multi-drug resistance protein 1 (MRP1) in EC109/Taxol cells.
We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/tcr-20-2678).
Methods
Materials and chemicals
2-ME (purity >98.0%) was obtained from Zhengzhou University (Zhengzhou, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Roswell Park Memorial Institute (RPMI)-1640 cell culture media were bought from Gibco Invitrogen. Dimethyl sulfoxide (DMSO) was obtained from Tianjin Deen Chemical Reagent Co., Ltd. (Tianjin, China). The water was pretreated with a Milli-Q-plus system (Millipore, Bedford, MA, USA). An annexin V-fluorescein isothiocyanate (FITC) propidium iodide (PI) apoptosis detection kit was purchased from Keygen Biotech. Co. (Cat No. KGA106, Nanjing, China). 5-Aza-2’-deoxycytidine (5-Aza-dC) was purchased from Sigma Aldrich Chemical Company (St. Louis, MO, USA). The nuclear-cytosol extraction kit and bicinchoninic acid (BCA) protein assay kits were purchased from Applygen Technologies Inc. The EpiQuiKTM DNA methyltransferase activity/inhibition assay kit was purchased from Epigentek. The human polyclonal anti-DNMT1, anti-P-gp, anti-BCRP, and anti-MRP1 antibodies were purchased from Abcam; β-actin antibodies were purchased from Cell Signaling Technology (Boston, MA, USA).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Cell line and cell culture
The human ESCC cell line EC109 was purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. It is passed down and preserved by our laboratory. ECl09/Taxol cell line: paclitaxel was used as an inducing drug, and human ESCC EC109 cells were used as parent cells. The method of high-dose intermittent induction of binding time was used to increase the binding time. It took 6 months to establish human ESCC. Paclitaxel resistant cell line EC109/Taxol.
The EC109 and EC109/Taxol cells were cultured in normal RPMI-1640 culture medium with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in 5% CO2 and 95% air at 37 °C in a humidified incubator (17).
Drug sensitivity assay
EC109 and EC109/Taxol cells (8×103 cells) were plated in 96-well plates and then incubated for 24 h. After incubating, the medium was removed and replaced with media containing various concentrations of 2-ME (0.5, 1, 2, 5, and 10 µM). In contrast, a medium without drug was added to the control and blank wells. The plates were incubated at 37 °C and 5% CO2 for 24, 48, and 72 h; a standard MTT assay was then used to investigate cytotoxicity (21,22).
Cell cycle analysis
EC109 and EC109/Taxol cells were seeded in 6-well plates (8×105 cells per well). Cells were treated with 2-ME (2, 5, and 10 µM) for 24 hours. Then the cells were trypsinized, washed twice with phosphate-buffered saline (PBS), and fixed at 4 °C overnight with cold ethanol (70%, V/V) (23). The fixed cells were washed and re-suspended in 500 µL PBS containing 50 µg/mL PI and 100 µg/mL RNase A. Samples were then incubated at 37 °C for 30 min in the dark and analyzed by flow cytometry (FCM, Cytomics FC 500 Beckman Coulter); cell populations in the G0/G1, S, and G2/M phase were quantified using Modifit LT software.
Cellular apoptosis assay
Cellular apoptosis was monitored using an annexin-V-Fluos staining kit (Sigma Aldrich Co. LLC). EC109 and EC109/Taxol cells were treated with 2-ME (2, 5, and 10 µM) for 24 hours. The cells were then trypsinized, washed with PBS, and re-suspended in 500 µL binding buffer. After adjusting cell density to 1×106 cells/mL, the samples were incubated with an additional 5 µL annexin V and 5 µL PI for 15 min at room temperature in the dark. The cells were immediately analyzed using FCM (BD Accuri C6). Annexin V-FITC-positive cells represent the early apoptotic populations. Annexin V-PI-positive cells represent either late apoptotic or secondary necrotic populations (24).
DNA ladder assay
An AllGen kit was used to extract DNA from apoptosis-induced cells according to the manufacturer’s instructions (25). To analyze DNA laddering, samples (3 µg per lane) were run using a 2% Agarose gel electrophoresis at 120 V for 2 h and then stained with ethidium bromide; the bands were then visualized under ultraviolet light and photographed (26).
DNMT activity assay
EC109 and EC109/Taxol cells were treated with 2-ME (2, 5, and 10 µM) and a universal DNMT inhibitor, 5-Aza-dC (10 µM), for 48 h respectively. Nuclear proteins were extracted using a nuclear-cytosol extraction kit (P1200). DNMT activity was measured using approximately 12 µg of nuclear protein and was measured using EpiQuiKTM DNA methyltransferase activity/inhibition assay kit according to the manufacturer’s instructions (27,28).
Western blot
After treating EC109 and EC109/Taxol cells with 2-ME (2, 5, and 10 µM) and 5-Aza-dC (10 µM), cells were gained, washed twice with PBS, and then separately incubated in RIPA buffer containing the phenylmethylsulfonyl fluoride (PMSF) protease inhibitor for 30 minutes on ice. The insoluble debris was precipitated by centrifugation at 12,000 rpm at 4 °C for 15 minutes; the supernatant was collected, and the protein concentration was measured using a BCA protein assay kit. Western blots were performed using standard protocols. The levels of DNMT1, P-gp, BCRP, and MRP1 proteins were analyzed with the following antibodies: human polyclonal anti-DNMT1, anti-P-gp, anti-BCRP, and anti-MRP1 (1:500) respectively. Then enhanced chemiluminescence (ECL) Western blot detection reagents were performed (29-31).
Statistical analysis
All experiments described above were independently performed at least 3 times. Quantitative data are expressed as means ± standard deviation (SD) and were analyzed using a paired-sample t-test (29). Furthermore, P values <0.05 were considered statistically significant.
Results
2-ME-mediated inhibition of EC109/Taxol cell proliferation
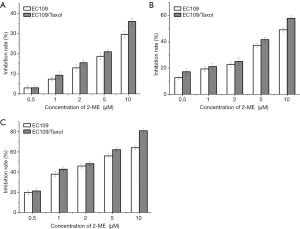
To determine whether 2-ME increases cytotoxicity in EC109/Taxol cells, EC109/Taxol cells, and the EC109 parental cells were treated with 2-ME at different concentrations for 24, 48, and 72 h respectively. As is shown in Figure 1. The inhibition rates of EC109/Taxol were 3.04%±1.21%, 9.34%±1.73%, 15.58%±1.25%, 21.05%±1.28% and 36.18%±1.42%, respectively, at the concentration of 2-ME at 0.5, 1, 2, 5, 10 mol/L for 24 h. The inhibition rates of the EC109 parental cells were 2.96±1.13, 7.39±1.01, 12.97±1.62, 18.77±1.63, 29.72±1.47, respectively. The inhibition rates of EC109/Taxol cells were 17.23%±2.61%, 21.17%±2.42%, 25.03%±3.11%, 41.72%±1.68%, 57.96%±2.17%, respectively, when the concentration of 2-ME was 0.5, 1, 2, 5, 10 mol/L for 48 h. The inhibition rates of the EC109 parental cells were 12.73±1.13, 19.37±2.21, 22.76±2.00, 37.33, 49.18±1.86, respectively. The inhibition rates of EC109/Taxol were 21.29%±2.61%, 42.87%±2.22%, 48.34%±2.09%, 62.23%±1.80% and 80.99%±2.12%, respectively, when the concentrations of 2-ME were 0.5, 1, 2, 5, 10 mol/L for 72 h. The inhibition rates of the EC109 parental cells were 19.89±2.13, 37.97±2.71, 45.96±2.04, 56.03±1.98 and 64.22±1.86 respectively. 2-ME significantly inhibited EC109/Taxol and EC109 cell viability in a time- and dose-dependent manner, with IC50 values of 2.04 and 5.38 µM after 72-h treatment respectively.
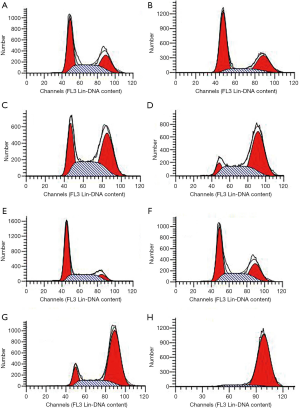
2-ME increased G2/M cell cycle arrest
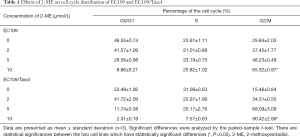
Previously, it was reported that the proportion of EC109/Taxol cells in the G0/G1 and S phases was significantly higher than that of EC109 cells, with a concomitant significant reduction in the G2/M phase (11). To determine whether 2-ME affects cell cycle resistance, the distribution of cell cycle in EC109/Taxol and EC109 cells that are treated by 2-ME were evaluated. The results (Figure 2 and Table 1) show that treatment with different concentrations of 2-ME for 24 h induced a similar G2/M cell cycle arrest in these two cell lines; however, the effect on EC109/Taxol cells was significantly obvious than that on EC109 cells trial with 2-ME in 10 µmol/L [DF =2, F(0.050, 2.056) =16.518, P=0.046, Figure 2 and Table 1] (30).
Full table
2-ME-induced apoptosis in EC109/Taxol cells
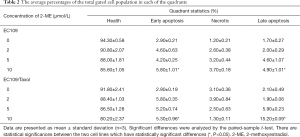
To test whether 2-ME changes apoptosis in a dose-dependent manner, we assessed 2-ME-treatment in EC109/Taxol cells and EC109 cells using an annexin V-PI double-staining method. As is shown in Table 2, spontaneous apoptosis (early and late apoptotic cells) was less than 10% in untreated cells; however, approximately 10% of EC109 cells and 20% of EC109/Taxol cells underwent apoptosis when incubated with 2-ME in 10 µmol/L [DF =2, F(1.079, 3.072) =16.302, P=0.026, Table 2].
Full table
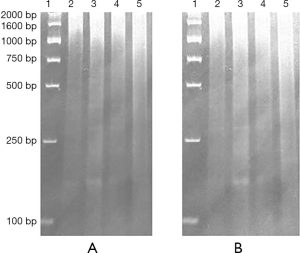
DNA ladder from 2-ME-induced cellular apoptosis
To further determine cellular apoptosis, apoptosis marker, and DNA fragmentation was detected (31,32). To study the cyto-protective effects of 2-ME in terms of apoptosis, DNA ladders were examined. After treatment with 2-ME (10 µM) for 48 h, DNA degradation ladders (consisting of multiple fragments of approximately 180–200 bp in length) were observed in EC109/Taxol cells and EC109 cells in 2% Agarose gel electrophoresis, as is shown in Figure 3. However, the DNA smear was weak and no DNA degradation ladders were observed in cells treated with 2 and 5 µM 2-ME or the blank control cells.
2-ME inhibited DNMT enzymatic activity
DNMT enzymatic activity was determined in nuclear extracts of EC109 and EC109/Taxol cells respectively. Both EC109 and EC109/Taxol cells were treated with various concentrations of 2-ME for 72 h, and 5-Aza-dC treatment was used as a hypomethylation control. The results showed that nuclear extracts from these two cell lines were significantly reduced when both cell lines were treated with 10 µM concentrations of 2-ME [DF =2, F(0.097, 0.756) =5.565, P=0.031, Figure 4A]. These results indicate that perhaps the 2-ME interferes with the nuclear extraction technique. In addition, nuclear extracts from EC109/Taxol cells treated with the same concentration of 2-ME were less than those from the parental EC109 cells.
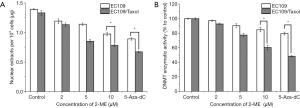
From the results of the amount of nucleoprotein, the amount of nucleoprotein extracted from the blank EC109/Taxol. Every 104 cells in the blank EC109/Taxol cells were significantly lower than that of its parent the blank EC109 cells. With the increase of 2-ME drug concentration, the amount of nucleoprotein extracted was significantly reduced, and at the same 2-ME concentration, the EC109/Taxol cell group had less nucleoprotein extracted than its parent cell group. It is very likely that the expression level of DNMTs in the blank EC109/Taxol cells is higher than that of its parental cells, making the amount of nuclear protein extracted per 1×104 cells in the blank EC109/Taxol cells significantly lower than its parent cell. As the concentration of 2-ME drug increases, the amount of nucleoprotein extracted is significantly reduced. It is very likely that 2-ME reduces the expression of DNMTs by inhibiting the activity of DNMTs, thereby affecting the amount of overall nucleoprotein.
From the result of the activity of the DNMT enzyme, as the concentration of 2-ME drug increases, the activity of DNMTs is significantly reduced in both EC109/Taxol cells and its parent cells. Furthermore, it can be proved that 2-ME reduces the expression of DNMTs by inhibiting the activity of DNMTs, thereby affecting the amount of overall nuclear protein.
In summary, the experiments in this chapter show that 2-ME can significantly reduce the activity of DNMTs.
Moreover, we assessed global DNMT activity levels using the EpiQuiKTM DNA methyltransferase activity/inhibition assay kit (Figure 4B). The result of the assessment indicated that 10 µM concentrations of 2-ME exposure could significantly decrease DNMT enzymatic activity in EC109/Taxol cells when compared to EC109 [DF =2, F(0.054, 0.111) =6.318, P=0.024, Figure 4].
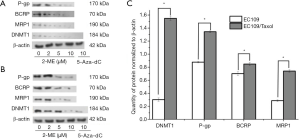
DNMT1 and MDR-associated protein expression is downed-regulated in EC109/Taxol cells treated with 2-ME
According to some reports, the up-regulation of DNMT1 accounts for the drug resistance phenotype (33,34). Differences in the expression of DNMT1 between the EC109 and EC109/Taxol cells were determined. The results (Figure 5) show that the expression of DNMT1 is higher in EC109/Taxol cells than that in EC109 cells with 2-ME. The effects of 2-ME and 5-Aza-dC on DNMT1 protein expression in EC109 and EC109/Taxol cells were compared. The results show that 2-ME significantly inhibits the expression of DNMT1. In addition, after treatment with 10 µM 2-ME for 72 h, expression of P-gp [DF =2, F(1.575, 9.755) =17.665, P=0.003], BCRP [DF =2, F(0.142, 0.309) =6.484, P=0.023], and MRP1 [DF =2, F(0.227, 0.758) =7.991, P=0.015] decreased in EC109/Taxol cells, which was statistically significant differences. The results show that the DNMT1, P-gp, BCRP, and MRP1 protein expression ratios of EC109/Taxol to EC109 cells are respectively shown in the figure (Figure 2E).
Discussion
EC is one of the worst malignant digestive neoplasms, because of its high malignant potential and poor prognosis (35). Treatment strategies for esophageal malignancies can conceptually be divided along two axes: locoregional treatment and systemic therapy. Even though EC can be treated by surgical techniques, many patients succumb to distant metastases after curative resection. It has been demonstrated that EC’s sensitivity to chemotherapy and radiotherapy is higher when compared with other gastrointestinal cancers (36). Twelve drugs are approved by FDA for EC, nine of which have clinical biomarkers associated with either resistance (37). Among them, paclitaxel is used to treat EC expressing tubulin beta biomarkers (37,38). As a chemotherapeutic drug, meanwhile, the emergence of paclitaxel resistance greatly affects its impact and restricts its application in the clinic (9). Interestingly, at pleiotropic pharmacological, documentaries show that 2-ME was well tolerated, and by inhibiting the growth, metastasis, and angiogenesis of tumor cells, it shows excellent safety without serious toxicity (12-16). Numerous angiogenic genes can be regulated by 2-ME directly or indirectly. The studies in oncology, 2-ME exhibited anti-angiogenic activity in the corneal micropocket vascularization in vivo and inhibited development of neovascularization in rat aortic ring assay (39).
Chemotherapy is the main strategy for the treatment of cancer. However, the main problem limiting the success of chemotherapy is the development of MDR. Exact elucidation of resistance mechanisms and molecular and biochemical approaches to overcome MDR have been a major goal in cancer research. It is reported that the hypermethylation of tumor suppressor genes with normal structure mediates the transcriptional silencing of these genes, thereby greatly promoting the occurrence or development of cancer (40-42). Postreplicative modification by DNA methylation in prokaryotic and eukaryotic genomes has been reported to be involved, including the regulation of gene expression, inactivation of the X-chromosome, and preservation of chromosomal integrity, in a variety of vital biological functions. The current field of MDR is a major problem due to the lack of non-toxic and specific compounds. At present, studies have found that inhibiting the activity of three major ABC transporters (P-gp or MDR1, MRP1 or ABCC1, MRP2 and BCRP or ABCG2) may become a hot spot in the exploration of high-efficiency and low-toxicity multi-drug resistance reversers (43). In addition to proposing various prodrug strategies to make paclitaxel more effective, the combined administration can improve the chemotherapy effect of paclitaxel, which has an important clinical application value (44). Hypothesis tumor progression was inhibited through the combination of 2-ME and Paclitaxel regulating DNMT activity up-regulate recessive oncogene expression. The study of DNMT activity showed that the volume of nuclear extract from these two cell lines was significantly reduced in a dose-dependent manner after treatment with various concentrations of 2-ME, which is presented in Figure 4. In addition, the amount of nuclear extract from EC109/Taxol cells treated with the same concentration of 2-ME was less than that observed in the EC109 parental cells, which can be seen in Figure 4. The decrease in DNMT activity might lead to an increase in apoptosis. The decrease in DNMT activity might change pharmacological action in the cell cycle and apoptosis due to paclitaxel and 2-ME.
When EC109 treated by 2-ME and paclitaxel, changes in cell cycle and apoptosis were also observed. Paclitaxel is an anticancer drug that can promote the polymerization of tubulin and stabilize the depolymerization of microtubules (45). The mechanism research showed, at low concentrations, that paclitaxel inhibits the depolymerization of microtubules and apoptosis is induced at G0 and G1/S phase. At high concentrations, paclitaxel increases the number and quality of microtubules, thus increasing the stability of microtubules. By blocking the detachment of microtubule minus ends from centrosomes, paclitaxel could disable the function of microtubules, and the mitotic cycle stopped at the G2/M phase (45-48). The induction of apoptosis by paclitaxel may not only depend on the concentration, but also the duration of exposure (47,48). Previous studies have found that paclitaxel at a concentration of n nm in EC109 has some cytotoxic effect. In this study, it is observed that after EC109 parent cells and EC109/Taxol cells were treated with 2-ME, the viability of EC109/Taxol cells was significantly inhibited. In addition, cell viability was inhibited in a time- and dose-dependent manner, with IC50 values of 2.04 and 5.38 µM following treatment for 72 h, respectively. Cell cycle arrest also was observed. As is shown in Figure 2 and Table 1, treatment with different concentrations of 2-ME for 24 h could induce similar G2/M cell cycle arrest in these two cell lines; however, the effect in EC109/Taxol cells was more obvious than that in EC109 cells (P<0.05). DNA fragmentation further proved cellular apoptosis. These data in Figure 3 collectively demonstrated that 2-ME inhibits the growth of EC109/Taxol and EC109 parent cells by inducing apoptosis. This phenomenon may be due to the combined use of 2-ME and paclitaxel enhances cytotoxicity, which caused apoptosis and led to cell cycle arrest (45-48). This phenomenon also involved the expression of multiple resistance proteins (49-51).
Paclitaxel also exerts its mechanism of action by activation of multiple signal-transduction pathways, including the Toll-like receptor 4 (TLR4) dependent pathway, c-Jun N-terminal kinase (JNK), p38 mitogen activated protein (MAP) Kinase and nuclear factor kappa B (NF-κB). These mechanisms may be associated with cytokines and pro-inflammatory proteins. Paclitaxel may have a leading immunomodulatory effect at low doses, but it may cause cell death at high doses (49,52-54). However, paclitaxel is also faced with multiple drug resistance like other anti-cancer agents (44). One of the mechanisms were involved in drug efflux from cancer cells is mediated by ABC transporters such as P-gp (49-51). DNMT1 is the major methyltransferase in mammals. It is shown that some cancer developments might be associated with DNMT1-mediated DNA methylation (55,56). Western blot analysis revealed that the expression of ABC transporters, including P-gp, BCRP, DNMT1, and MRP1, in EC109/Taxol cells was much higher than that in EC109 cells. Further, they were correspondingly down-regulated by 2-ME, which is shown in Figure 5.
In conclusion, the combination treatment of non-toxic concentration of 2-ME and paclitaxel can induce cytotoxicity in EC109 cells by regulating the expression of DNMT1 and the three main ABC transporters, which may be used as an adjuvant for EC chemotherapy. Further studies in vitro and in vivo are needed to explain the modulation mechanisms, which will provide a better opportunity for the treatment of EC.
Acknowledgments
Funding: The study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/tcr-20-2678
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-2678
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2678). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kosumi K, Baba Y, Sakamoto A, et al. Lysine-specific demethylase-1 contributes to malignant behavior by regulation of invasive activity and metabolic shift in esophageal cancer. Int J Cancer 2016;138:428-39. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Lambert R. Endoscopy in screening for digestive cancer. World J Gastrointest Endosc 2012;4:518-25. [Crossref] [PubMed]
- Lin R, Li X, Li J, et al. Long-term cisplatin exposure promotes methylation of the OCT1 gene in human esophageal cancer cells. Dig Dis Sci 2013;58:694-8. [Crossref] [PubMed]
- O'Donovan TR, O'Sullivan GC, McKenna SL. Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. Autophagy 2011;7:509-24. [Crossref] [PubMed]
- Wang XX, Liu R, Jin SQ, et al. Overexpression of Aurora-A kinase promotes tumor cell proliferation and inhibits apoptosis in esophageal squamous cell carcinoma cell line. Cell Res 2006;16:356-66. [Crossref] [PubMed]
- Li Z, Zhou S, Zhang L, et al. BIM induction of apoptosis triggered by EGFR-sensitive and resistance cell lines of non-small-cell lung cancer. Med Oncol 2011;28:572-7. [Crossref] [PubMed]
- Liu J, Jiang Z, Xiao J, et al. Effects of triptolide from Tripterygium wilfordii on ERalpha and p53 expression in two human breast cancer cell lines. Phytomedicine 2009;16:1006-13. [Crossref] [PubMed]
- Wei Y, Pu X, Zhao L. Preclinical studies for the combination of paclitaxel and curcumin in cancer therapy Oncol Rep 2017;37:3159-66. (Review). [Crossref] [PubMed]
- Yusuf RZ, Duan Z, Lamendola DE, et al. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets 2003;3:1-19. [Crossref] [PubMed]
- Wang C, Guo LB, Ma JY, et al. Establishment and characterization of a paclitaxelresistant human esophageal carcinoma cell line. Int J Oncol 2013;43:1607-17. [Crossref] [PubMed]
- Harrison MR, Hahn NM, Pili R, et al. A phase II study of 2-methoxyestradiol (2ME2) NanoCrystal(R) dispersion (NCD) in patients with taxane-refractory, metastatic castrate-resistant prostate cancer (CRPC). Invest New Drugs 2011;29:1465-74. [Crossref] [PubMed]
- Bruce JY, Eickhoff J, Pili R, et al. A phase II study of 2-methoxyestradiol nanocrystal colloidal dispersion alone and in combination with sunitinib malate in patients with metastatic renal cell carcinoma progressing on sunitinib malate. Invest New Drugs 2012;30:794-802. [Crossref] [PubMed]
- Tevaarwerk AJ, Holen KD, Alberti DB, et al. Phase I trial of 2-methoxyestradiol NanoCrystal dispersion in advanced solid malignancies. Clin Cancer Res 2009;15:1460-5. [Crossref] [PubMed]
- Zou X, Zhou L, Zhu W, et al. Effectiveness of 2-methoxyestradiol in alleviating angiogenesis induced by intracranial venous hypertension. J Neurosurg 2016;125:746-53. [Crossref] [PubMed]
- Lin HL, Yang MH, Wu CW, et al. 2-Methoxyestradiol attenuates phosphatidylinositol 3-kinase/Akt pathway-mediated metastasis of gastric cancer. Int J Cancer 2007;121:2547-55. [Crossref] [PubMed]
- Zhang N, Xia Y, Guo X, et al. Preparation, characterization, and in vitro targeted delivery of folate-conjugated 2-methoxyestradiol-loaded bovine serum albumin nanoparticles. J Nanopart Res 2014;16:2390. [Crossref]
- Ehrlich M. DNA hypermethylation in disease: mechanisms and clinical relevance. Epigenetics 2019;14:1141-63. [Crossref] [PubMed]
- Vijayaraghavalu S, Labhasetwar V. Efficacy of decitabine-loaded nanogels in overcoming cancer drug resistance is mediated via sustained DNA methyltransferase 1 (DNMT1) depletion. Cancer Lett 2013;331:122-9. [Crossref] [PubMed]
- Li S, Zhang W, Yin X, et al. Mouse ATP-Binding Cassette (ABC) Transporters Conferring Multi-Drug Resistance. Anticancer Agents Med Chem 2015;15:423-32. [Crossref] [PubMed]
- Azab SS, Salama SA, Abdel-Naim AB, et al. 2-Methoxyestradiol and multidrug resistance: can 2-methoxyestradiol chemosensitize resistant breast cancer cells? Breast Cancer Res Treat 2009;113:9-19. [Crossref] [PubMed]
- Du B, Zhao Z, Sun H, et al. Effects of 2-methoxyestradiol on proliferation, apoptosis and gene expression of cyclin B1 and c-Myc in esophageal carcinoma EC9706 cells. Cell Biochem Funct 2012;30:158-65. [Crossref] [PubMed]
- Zhou L, Hou J, Fu W, et al. Arsenic trioxide and 2-methoxyestradiol reduce beta-catenin accumulation after proteasome inhibition and enhance the sensitivity of myeloma cells to Bortezomib. Leuk Res 2008;32:1674-83. [Crossref] [PubMed]
- Zhou X, Jiang H, Hou J. Coordination of upregulated XBP-1 and downregulated c-myc during myeloma cell differentiation induced by 2-methoxyestradiol. Leuk Res 2007;31:1259-65. [Crossref] [PubMed]
- Ebrahimisadr P, Ghaffarifar F, Mohammad Hassan Z. In-vitro Evaluation of Antileishmanial Activity and Toxicity of Artemether with Focus on its Apoptotic Effect. Iran J Pharm Res 2013;12:903-9. [PubMed]
- Brocca A, Virzi GM, de Cal M, et al. Cytotoxic effects of p-cresol in renal epithelial tubular cells. Blood Purif 2013;36:219-25. [Crossref] [PubMed]
- Chen Z, Pan X, Yao Y, et al. Epigenetic regulation of cardiac progenitor cells marker c-kit by stromal cell derived factor-1alpha. PLoS One 2013;8:e69134. [Crossref] [PubMed]
- Pan X, Chen Z, Huang R, et al. Transforming growth factor beta1 induces the expression of collagen type I by DNA methylation in cardiac fibroblasts. PLoS One 2013;8:e60335. [Crossref] [PubMed]
- Shi J, Zhang H, Wang L, et al. PEI-derivatized fullerene drug delivery using folate as a homing device targeting to tumor. Biomaterials 2013;34:251-61. [Crossref] [PubMed]
- Choi HJ, Zhu BT. Critical role of cyclin B1/Cdc2 up-regulation in the induction of mitotic prometaphase arrest in human breast cancer cells treated with 2-methoxyestradiol. Biochim Biophys Acta 2012;1823:1306-15. [Crossref] [PubMed]
- Khodarev NN, Sokolova IA, Vaughan AT. Mechanisms of induction of apoptotic DNA fragmentation. Int J Radiat Biol 1998;73:455-67. [Crossref] [PubMed]
- Yu J, Ye J, Liu X, et al. Protective effect of L-carnitine against H(2)O(2)-induced neurotoxicity in neuroblastoma (SH-SY5Y) cells. Neurol Res 2011;33:708-16. [Crossref] [PubMed]
- Gravina GL, Marampon F, Piccolella M, et al. Hormonal therapy promotes hormone-resistant phenotype by increasing DNMT activity and expression in prostate cancer models. Endocrinology 2011;152:4550-61. [Crossref] [PubMed]
- Mishra MV, Bisht KS, Sun L, et al. DNMT1 as a molecular target in a multimodality-resistant phenotype in tumor cells. Mol Cancer Res 2008;6:243-9. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Sohda M, Kuwano H. Current Status and Future Prospects for Esophageal Cancer Treatment. Ann Thorac Cardiovasc Surg 2017;23:1-11. [Crossref] [PubMed]
- Abdo J, Agrawal DK, Mittal SK. Basis for molecular diagnostics and immunotherapy for esophageal cancer. Expert Rev Anticancer Ther 2017;17:33-45. [Crossref] [PubMed]
- FDA updates labeling for erectile dysfunction drugs. FDA Consum 2005;39:3. [PubMed]
- Zou X, Zhang L, Yuan J, et al. Endogenous hormone 2-methoxyestradiol suppresses venous hypertension-induced angiogenesis through up- and down-regulating p53 and id-1. J Cell Mol Med 2018;22:957-67. [PubMed]
- Garzon R, Liu S, Fabbri M, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood 2009;113:6411-8. [Crossref] [PubMed]
- Laird PW, Jaenisch R. The role of DNA methylation in cancer genetic and epigenetics. Annu Rev Genet 1996;30:441-64. [Crossref] [PubMed]
- Jung G, Hernández-Illán E, Moreira L, et al. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol 2020;17:111-30. [Crossref] [PubMed]
- Kumar A, Jaitak V. Natural products as multidrug resistance modulators in cancer. Eur J Med Chem 2019;176:268-91. [Crossref] [PubMed]
- Meng Z, Lv Q, Lu J, et al. Prodrug Strategies for Paclitaxel. Int J Mol Sci 2016;17:796. [Crossref] [PubMed]
- Ganguly A, Yang H, Cabral F. Paclitaxel-dependent cell lines reveal a novel drug activity. Mol Cancer Ther 2010;9:2914-23. [Crossref] [PubMed]
- Wang F, Cao Y, Zhao W, et al. Taxol inhibits melanoma metastases through apoptosis induction, angiogenesis inhibition, and restoration of E-cadherin and nm23 expression. J Pharmacol Sci 2003;93:197-203. [Crossref] [PubMed]
- Sevko A, Kremer V, Falk C, et al. Application of paclitaxel in low non-cytotoxic doses supports vaccination with melanoma antigens in normal mice. J Immunotoxicol 2012;9:275-81. [Crossref] [PubMed]
- Giannakakou P, Sackett DL, Kang YK, et al. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem 1997;272:17118-25. [Crossref] [PubMed]
- Yakirevich E, Sabo E, Naroditsky I, et al. Multidrug resistance-related phenotype and apoptosis-related protein expression in ovarian serous carcinomas. Gynecol Oncol 2006;100:152-9. [Crossref] [PubMed]
- Gottesman MM, Ludwig J, Xia D, et al. Defeating drug resistance in cancer. Discov Med 2006;6:18-23. [PubMed]
- Yang X, Iyer AK, Singh A, et al. Cluster of Differentiation 44 Targeted Hyaluronic Acid Based Nanoparticles for MDR1 siRNA Delivery to Overcome Drug Resistance in Ovarian Cancer. Pharm Res 2015;32:2097-109. [Crossref] [PubMed]
- Pfannenstiel LW, Lam SS, Emens LA, et al. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol 2010;263:79-87. [Crossref] [PubMed]
- Szakács G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006;5:219-34. [Crossref] [PubMed]
- Wang TH, Chan YH, Chen CW, et al. Paclitaxel (Taxol) upregulates expression of functional interleukin-6 in human ovarian cancer cells through multiple signaling pathways. Oncogene 2006;25:4857-66. [Crossref] [PubMed]
- Du Z, Ma K, Sun X, et al. Methylation of RASSF1A gene promoter and the correlation with DNMT1 expression that may contribute to esophageal squamous cell carcinoma. World J Surg Oncol 2015;13:141. [Crossref] [PubMed]
- Li H, Liu JW, Sun LP, et al. A Meta-Analysis of the Association between DNMT1 Polymorphisms and Cancer Risk. Biomed Res Int 2017;2017:3971259. [Crossref] [PubMed]


