
Effect of selective lymph node dissection on immune function in patients with T1 stage non-small cell lung cancer: a randomized controlled trial
Introduction
Lung cancer is a common clinical malignant tumor. Non-small cell lung cancer (NSCLC) accounts for approximately 80% of lung cancer. Among all malignant tumors, the incidence and mortality of lung cancer rank first in China and across the world. At present, surgery is still the first choice for treating lung cancer, and the dissection of metastatic lymph nodes plays a key role in improving patients’ disease-free survival (DFS) and overall survival (OS) (1). Selective lymph node dissection (SLND) refers to the selective removal of intrapulmonary lymph nodes, hilar lymph nodes, and mediastinal lymph nodes, as well as surrounding adipose tissue, which easily metastasize depending on the location of the primary tumor (2). Ishiguro et al. (3) showed that SLND can reduce perioperative complications and protect the immune function of patients after NSCLC, but does not reduce the survival rate of patients.
Surgery has dual effects on the immune function of lung cancer patients. On the one hand, surgery can completely remove the tumor and relieve tumor-derived immunosuppression (4,5). On the other hand, surgical trauma puts patients in a state of stress and weakens the body’s cellular immune function (6,7). Changes in T cells subsets and NK cells in the peripheral blood of lung cancer patients are important indicators reflecting the body’s cellular immune function (8).
For some early-stage NSCLC patients, SLND can achieve the same effect as systematic lymph node dissection in removing metastatic lymph nodes. Whether SLND can reduce damage to immune function and accelerate the rapid recovery of patients after surgery requires further study. This study mainly compared the clinical indicators and immune indicators of the two groups of patients who underwent systemic lymph node dissection and SLND, and further demonstrated whether SLND can reduce the damage to the immune function of surgery and facilitate the rapid recovery of patients after surgery. We present the following article in accordance with the CONSORT reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-524).
Methods
Research subjects and grouping method
This study has been registered in Chinese Clinical Trial Registry, the registration title is: Effect of selective lymph node dissection on immune function in patients with T1 stage non-small cell lung cancer, the registration number is: ChiCTR2100045893, the registration institution is: Linyi People’s Hospital.
From July 2018 to June 2019, patients who underwent surgical treatment in our hospital and had lung tumors of diameters less than 3 cm were selected as subjects. Informed consent was obtained from the patient or the patient’s family before surgery. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of Linyi People’s Hospital (NO.: YX30066) and informed consent was taken from all the patients. The lung tumor along with hilar and pulmonary regional lymph nodes were excised and sent for fast frozen pathology during the operation (lymph nodes were sent alone if the tumor had definite pathology before surgery). If the lung tumor was benign or the hilar/pulmonary regional lymph nodes had cancer metastasis, the patient was excluded from the study. Only patients with NSCLC who had no metastases in the hilar and pulmonary regional lymph nodes were used as the study subjects. These patients were randomly divided into 2 groups: SLND (Group SD) and systematic lymph node dissection (Group CD). Finally, there were 32 patients in Group SD and 38 patients in Group CD.
The inclusion criteria were as follows: (I) age ≤75 years; (II) single tumor with diameter ≤3 cm; (III) enhanced chest CT or PET-CT showed no obvious mediastinum and hilar lymphadenopathy (maximum diameter of the lymph nodes <1 cm); (IV) before or during surgery patients were clearly identified as having NSCLC; (V) preoperative examination showed that patients could tolerate thoracoscopy lobectomy; (VI) patients and their families agreed to participate in the study.
The exclusion criteria were as follows: (I) concomitant tumors; (II) received neoadjuvant chemoradiotherapy; (III) lung tumor was located in the right middle lobe; (IV) recent history of infections and autoimmune diseases or oral hormones or drugs that affect immune function; (V) history of thoracic surgery.
The elimination criteria were as follows: (I) confirmed non-NSCLC patients; (II) lymph node metastasis confirmed by frozen pathology; (III) patients who underwent thoracotomy; (IV) intraoperative blood loss >200 mL; (V) sublobar resection; (VI) temperature >38.5 °C after surgery, severe infection, long-term air leakage (≥7 days); (VII) severe complications such as ventilator-assisted breathing, pulmonary embolism, or secondary thoracotomy.
Observation indicators
Relevant indicators were measured 24 hours before surgery and on the 1st and 3rd postoperative days (POD), including: (I) cytokine indexes: IL-6, CRP; (II) cellular immune indexes: lymphocytes, NK cells, CD4+, CD8+, CD4+/CD8+; (III) humoral immune indexes: IgG, IgA, IgM; (IV) clinical indexes: operation time, intraoperative blood loss, pleural effusion drainage volume, thoracic drainage tube retention time, postoperative hospital-stay, and postoperative complications.
Research methods
Specimen collection
Patients who met the inclusion criteria and were determined to undergo surgery had venous blood drawn on the morning before surgery to detect CRP, IgG, IgA, IgM, lymphocytes, NK cells, CD4+, CD8+, and CD4+/CD8+. At the same time, approximately 2.5 mL venous blood was drawn, left for 10–20 minutes, then centrifuged (2,000–3,000 rpm, 20 min). The serum was collected and stored in a refrigerator at −20 °C. For enrolled patients, venous bloods were collected on POD1 and POD3 in the same way as before, and related indicators were tested and serum specimens were obtained. After all the specimens were collected, IL-6 in serum was tested.
Specimen testing
IgG, IgA, IgM, CRP detection methods and procedures
Immune Scattering Turbidimetric Method: (I) Detection principle. The protein in the human body fluid sample will form an immune complex with specific antibodies. These immune complexes scatter the light beam passing through the specimen. The intensity of the scattered light is proportional to the concentration of related proteins in the specimen. The result can be obtained by comparing with the known standard concentration. (II) Measurement steps: Put the received blood sample in a centrifuge, centrifuge at 3,000 rpm for 10 minutes, take 500 µL of plasma into the automatic protein analyzer, and match the corresponding IgG, IgA, IgM, CRP kits, the test is performed separately, and the machine automatically calculates the result.
NK cells, CD4+, CD8+, CD4+/CD8+ detection methods and procedures
Flow cytometry method:
- Add 5 µL of fluorescently labeled antibody to the flow test tube.
- Add 50 µL of the mixed cell suspension, mix gently on a shaker, and incubate in the dark at room temperature for 15–20 minutes.
- Add 400 µL of red blood cell lysate to the incubated cells, shake to mix, and incubate for 10 minutes at room temperature in the dark.
- Add 3 mL PBS, shake and mix.
- Centrifugation at room temperature: 1,500 rpm, 5 minutes.
- Discard the supernatant and absorb the remaining liquid with absorbent paper.
- Add 400 µL PBS to resuspend the obtained cell pellet.
- Filter the cell suspension with a 300-mesh filter, check it with a flow cytometer, and the machine automatically calculates the result.
IL-6 detection methods and steps
After all the patients’ supernatants were collected, the IL-6 content was determined with the IL-6 determination kit, and the operation methods and steps were carried out in strict accordance with the kit instructions.
Lymphocyte detection methods and steps
After collecting a tube of anticoagulant sample, send it to our hospital’s laboratory. The laboratory physician will complete the routine blood test and record the lymphocyte value.
Surgical methods
Operations were performed by the same surgical team. The surgical method was VATS anatomical lobectomy + lymph node dissection with a single operation hole (9). According to the tumor location during the operation, lung tumors and hilar and pulmonary regional lymph nodes (right upper lobe: #2R, #3, #4R, #10, #11; left upper lobe: #4L, #5, #6, #10, #11; double lower lobe: #7, #8, #9, #10, #11) were removed and sent for fast frozen pathology (only lymph nodes were sent if the tumor had pathology before surgery). If the tumor was NSCLC, and no cancer cells were found in all lymph nodes removed, the random lottery method was used by the visiting nurse to divide the patient into Group SD or Group CD. Patients in Group SD only underwent SLND, while patients in Group CD continued to undergo resection of the remaining lymph nodes following systematic lymph node dissection criteria (left: #4L, #5, #6, #7, #8, #9, #10, #11; right: #2R, #3, #4R, #7, #8, #9, #10, #11). Patients were excluded from the study if the tumor was not NSCLC or the hilar/regional lymph nodes were metastatic.
Postoperative patient management
ECG monitoring, oxygen inhalation, and infection prevention were performed after surgery. Patients were transferred out of the monitoring room, and bedside chest radiographs and blood-related indicators were reviewed on POD1. If the lung was inflated, had no air leakage, and the 24-hour pleural drainage was less than 200 mL of non-blood fluid, the thoracic drainage tube was removed. Patients were discharged if there was no observed fever or discomfort 1 day after extubation. During hospitalization, the postoperative pleural effusion drainage volume, thoracic drainage tube retention time, postoperative hospital-stay, and postoperative complications were recorded.
Statistical methods
SPSS software 20.0 was used for statistical analysis (SPSS, Chicago, Illinois, USA). Measurement data is expressed as . Comparisons between groups were performed using the independent sample t test, and comparisons within groups were performed using the paired t test. Categorical variables were compared using the chi-square test or Fisher’s exact test. With 95% as the confidence interval, P<0.05 was considered statistically significant.
Results
Comparison of general clinical data between the 2 groups
A total of 150 patients were selected in this study, after inclusion criteria, exclusion criteria, and patients who were excluded from the experiment because they were not suitable for this study, a total of 75 patients were randomly divided into two groups, among them, 5 patients were excluded from this study because of adverse events (1 patient with fever and 1 patient with intraoperative bleeding in Group SD; 2 patients with fever and 1 patient with long-term air leakage in Group CD). In the end, there were 32 patients in the SD group and 38 patients in the CD group (Figure 1). Detailed information is shown in Table 1. There were no statistical differences in gender, age, tumor location, tumor diameter, and postoperative pathological type between the 2 groups (P>0.05). In terms of the number of lymph node dissections, Group SD had significantly less than Group CD (P<0.05).
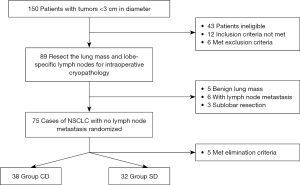
Table 1
| Group | N | Age (year) | Tumor diameter (cm) | Gender (male/female) | Tumor location (RUL/RLL/LUL/LLL) | LN dissection (N) | Pathologic types (AD/SC/other) |
|---|---|---|---|---|---|---|---|
| SD | 32 | 56.03±11.17 | 1.78±0.53 | 13/19 | 10/8/7/7 | 9.52±4.67 | 27/4/1 |
| CD | 38 | 58.40±9.97 | 1.84±0.61 | 16/22 | 11/10/9/8 | 15.68±8.21 | 30/7/1 |
| t/χ2 | 0.938 | 0.381 | 0.016 | 0.073 | 3.150 | 0.465 | |
| P | 0.352 | 0.704 | 0.900 | 0.995 | 0.002 | 0.792 |
N, number; LN, lymph node; RUL, right upper lung; RLL, right lower lung; LUL, left upper lung; LLL, left lower lung; AD, adenocarcinoma; SC, squamous carcinoma.
Comparison of perioperative clinical indicators between the 2 groups
In terms of intraoperative blood loss, operation time, pleural effusion drainage volume, and postoperative hospital stay, the values of Group SD were significantly lower than those of Group CD (P<0.05). There was no significant difference in thoracic drainage tube retention time between the 2 groups (P=0.074) (Table 2).
Table 2
| Group | Number | IBL (mL) | OT (min) | TDTRT (d) | PEDV (mL) | PHS (d) |
|---|---|---|---|---|---|---|
| SD | 32 | 48.13±22.06 | 105.56±28.22 | 3.10±1.20 | 461.09±214.81 | 4.78±1.10 |
| CD | 38 | 61.05±17.21 | 146.18±36.93 | 3.61±1.15 | 711.45±311.46 | 6.21±1.86 |
| t | 2.753 | 2.753 | 1.815 | 3.841 | 3.979 | |
| P | 0.008 | 0.008 | 0.074 | 0.000 | 0.000 |
IBL, intraoperative blood loss; OT, operation time; TDTRT, thoracic drainage tube retention time; PEDV, pleural effusion drainage volume; PHS, postoperative hospital-stay.
Comparison of postoperative complications between the 2 groups
The incidence of postoperative complications in Group SD and Group CD were 12.5% and 18.42%, respectively. There was no significant difference between the 2 groups (P>0.05), however, the incidence of complications in Group SD was relatively lower (Table 3).
Table 3
| Group | N | Fever (%) | Pneumonia (%) | Air leakage (%) | Arrhythmia (%) | ARDS (%) | Total (%) |
|---|---|---|---|---|---|---|---|
| SD | 32 | 1 (3.13) | 0 (0.00) | 1 (3.13) | 1 (3.13) | 1 (3.13) | 4 (12.50) |
| CD | 38 | 2 (5.26) | 1 (2.63) | 1 (2.63) | 2 (5.26) | 1 (2.63) | 7 (18.42) |
| χ2 | 0.460 | ||||||
| P | 0.498 |
N, number; ARDS, acute respiratory distress syndrome.
Comparison of cytokines between the 2 groups
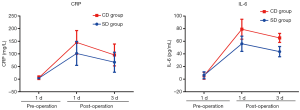
There were no significant differences in the levels of CRP and IL-6 between the 2 groups before surgery (P>0.05). CRP and IL-6 levels in both groups were significantly increased at POD1 and POD3 compared with levels before surgery (P<0.05), and the increase was more significant in Group CD than in Group SD (P<0.05) (Table 4 and Figure 2).
Table 4
| Group | Time | CRP (mg/L) | IL-6 (pg/mL) |
|---|---|---|---|
| SD (n=32) | Pre-op | 3.79±1.5 | 6.20±5.56 |
| POD1 | 101.98±47.46*# | 56.12±12.04*# | |
| POD3 | 67.11±39.45*# | 43.61±8.06*# | |
| CD (n=38) | Pre-op | 5.67±7.10 | 5.42±5.45 |
| POD1 | 145.51±46.98* | 79.14±15.80* | |
| POD3 | 95.88±43.22* | 65.38±7.16* |
Compared with preoperative levels, *P<0.05; compared with Group CD, #P<0.05. Pre-op, pre-operation; POD, postoperative days.
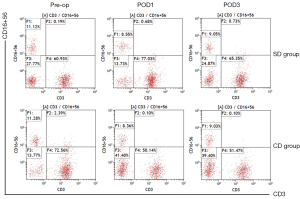
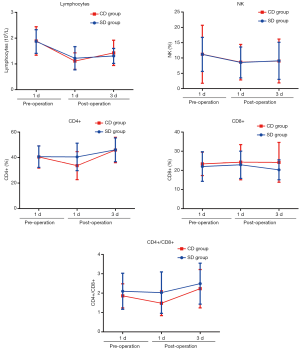
Comparison of cellular immune indexes between the 2 groups
The proportions of lymphocytes and NK cells at POD1 and POD3 in the 2 groups were significantly lower than those before surgery (P<0.05), however, there were no statistical differences between the 2 groups (P>0.05). The proportions of CD4+ cells and CD4+/CD8+ at POD1 in Group CD were significantly lower than those in Group SD (P<0.05), and the proportion of CD8+ cells at POD3 in Group SD was significantly lower than that in Group CD (P<0.05) (Table 5 and Figures 3-6).
Table 5
| Group | Time | Lymphocytes (109/L) | NK (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ |
|---|---|---|---|---|---|---|
| SD (n=32) | Pre-op | 1.87±0.46 | 11.17±5.56 | 40.69±8.43 | 22.02±7.73 | 2.10±0.93 |
| POD1 | 1.22±0.45* | 8.55±5.04* | 40.52±10.76# | 22.91±7.13 | 2.03±1.07# | |
| POD3 | 1.31±0.29* | 9.07±6.06* | 46.10±9.31 | 20.30±5.20# | 2.49±1.06 | |
| CD (n=38) | Pre-op | 1.89±0.55 | 11.22±9.45 | 40.48±8.69 | 23.38±6.11 | 1.86±0.62 |
| POD1 | 1.11±0.32* | 8.64±5.80* | 33.68±10.98 | 24.27±9.18 | 1.48±0.64 | |
| POD3 | 1.43±0.49* | 9.02 ±7.16* | 45.91±9.96 | 24.19±10.38 | 2.23±0.99 |
Compared with preoperative levels, *P<0.05; compared with Group CD, #P<0.05. Pre-op, pre-operation; POD, postoperative days.
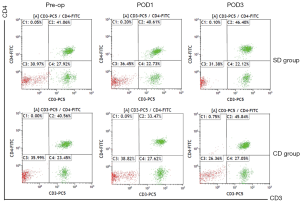
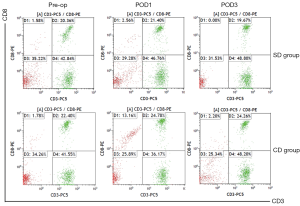
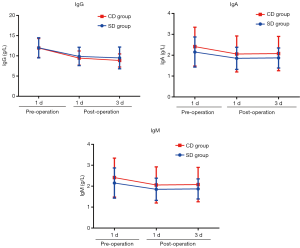
Comparison of humoral immunity indexes between the 2 groups
IgG level in the 2 groups was significantly decreased at POD1 and POD3 compared with that before surgery (P<0.05), while IgA and IgM levels were not significantly decreased (P>0.05). There were no significant differences in IgG, IgA, and IgM levels between the 2 groups at the same points (P>0.05) (Table 6 and Figure 7).
Table 6
| Group | Time | IgA (g/L) | IgG (g/L) | IgM (g/L) |
|---|---|---|---|---|
| SD (n=32) | Pre-op | 2.15±0.72 | 11.99±2.48 | 1.03±0.44 |
| POD1 | 1.85±0.53 | 9.85±2.28* | 0.83±0.37 | |
| POD3 | 1.87±0.48 | 9.49±2.70* | 0.81±0.37 | |
| CD (n=38) | Pre-op | 2.41±0.93 | 11.94±2.35 | 1.13±0.61 |
| POD1 | 2.06±0.86 | 9.45±1.76* | 0.91±0.47 | |
| POD3 | 2.08±0.82 | 8.86±1.61* | 0.88±0.44 |
Compared with preoperative levels, *P<0.05; compared with Group CD, #P<0.05. Pre-op, pre-operation; POD, postoperative days.
Discussion
Surgery has a dual effect on the patient’s immune function. (I) Surgery can eliminate the tumor microenvironment, thereby reducing immunosuppressive dendritic cells (4), and can reduce the secretion of inhibitory cytokines (TGFb, IL-10 and IL-35) from regulatory T cells (Treg), which can exert their immunosuppressive function on effector CD4+ T and CD8+ T cells (5). (II) Surgery can decrease the number of lymphocytes, thereby reducing the body’s cellular immune function; surgery can cause pro-inflammatory cytokines (TNF-α, IL-1, IL-3, IL-6, GM-CSF, G-CSF, IFN-γ) increased, and anti-inflammatory cytokines (IL-4, IL-10) decreased, thereby enhancing the inflammatory response, the cellular environment produced by systemic inflammation will destroy the normal apoptosis mechanism in activated immunocytes, thereby prolonging and enhancing the inflammatory response to injury (6,7). The trauma caused by surgery can trigger a series of acute stress reactions, which affect the body's immune function and mainly manifests as immunosuppression. For patients with malignant tumors, immunosuppression caused by surgical trauma can promote and accelerate the recurrence and metastasis of malignant tumors (10-12). In 1960, Professor Cahan (13) first proposed “systematic lymph node dissection”, and lobectomy plus systematic lymph node dissection became the standard surgical treatment for lung cancer (14,15). In recent years, the detection rate of early NSCLC has gradually increased. Lobectomy plus systematic lymph node dissection may aggravate the surgical trauma of many patients. Thoracic surgeons have been constantly exploring methods to ensure the complete resection of the tumor while minimizing surgical trauma. For example, thoracoscopy decreases the trauma caused by surgical incision, sublobectomy minimizes loss of lung function, and SLND and lymph node sampling has further reduced surgical trauma. Especially for patients with T1 stage NSCLC, SLND based on intraoperative lobular specific lymph node frozen pathology does not affect the prognosis of patients, but can reduce injury and postoperative complications (16).
Feasibility of SLND
SLND, also known as lobe-specific lymph node dissection (L-SLD), refers to the selective dissection of the hilum, regional lymph nodes, and surrounding mediastinal tissues based on the location of the primary tumor. SLND should first remove the hilum and regional lymph nodes of the lung, the lymph nodes are sent for intraoperative frozen pathology, then a decision is made whether or not to perform systematic lymph node dissection according to the intraoperative pathology. According to the location of the tumor, mediastinal lymph nodes prone to cancer metastasis are referred to as pulmonary regional lymph nodes, and hilar lymph nodes and pulmonary regional lymph nodes are referred to as lobe-specific lymph nodes. After reading the literature, we concluded the specific lymph node metastases areas: left upper lobe, hilum (#10, #11), and left superior mediastinum (#4L, #5, #6); left lower lobe, hilum (#10, #11), subcarinal (#7), and left inferior mediastinum (#8, #9); right upper lobe, hilum (#10, #11), and right superior mediastinum (#2R, #3, #4R); right lower lobe, hilum (#10, #11), subcarinal (#7), and right inferior mediastinum (#8, #9). The specific lymph node metastasis area of the right middle lobe is not obvious. Haruki et al. (17) found no hilar and mediastinal lymph node metastasis in patients with stage I NSCLC with predominantly ground glass nodules. Ohde et al. (18) and Hashizume et al. (19) showed that the 5-year survival rate of NSCLC patients with predominantly ground glass nodules less than 3 cm in diameter reached 95.7%, and systematic lymph node dissection was not required. Ishiguro et al. (3) found that patients who underwent SLND had shorter operation times, less intraoperative blood loss, and shorter hospital stays than patients who underwent systematic lymph node dissection. Multiple studies (3,16,20) have also shown that compared with systematic lymph node dissection, there were no significant differences in DFS, OS, and the postoperative recurrence rate, while the incidence of postoperative complications was significantly reduced.
Through a retrospective analysis of 841 patients with T1 stage NSCLC in our department (21), we found that for clinical T1 stage NSCLC patients, SLND based on the intraoperative fast frozen pathology of lobe-specific lymph nodes could achieve the same effect of tumor resection as systematic lymph node dissection.
In this study, we found that operation time, intraoperative blood loss, pleural effusion drainage volume, and postoperative hospital stay length in Group SD were significantly reduced compared with Group CD (P<0.05). There were no significant differences in the incidence of postoperative complications and thoracic drainage tube retention time between the 2 groups (P>0.05), but the values were lower in Group SD. The lack of a statistical difference may be related to the small sample size.
Effects of SLND on cytokines and non-specific immunity
Cytokines (CK) are multifunctional and highly active small molecules synthesized and secreted by a variety of cells (mainly immune cells). They mainly mediate and regulate inflammatory responses and immune responses, and are important regulatory molecules involved in the intrinsic and adaptive immune response.
The effect of surgical trauma on the body’s immune system firstly manifests as an acute inflammatory response, which is mainly marked by the release of TNF-α, IL-6, and CRP (22). IL-6 is a key cytokine with immunomodulatory functions, and is involved in acute phase response. It can be used as an early sensitive indicator of tissue damage, reflecting the severity of injury. IL-6 can regulate the levels of IL-1β and TNF-α in vivo, thus affecting cellular immunity and immune monitoring. In the early stages of cytokine activation, IL-6 has shown to be significantly associated with the incidence of postoperative complications and surgical mortality. Thoracoscopy significantly reduced the levels of pro-inflammatory cytokines (IL-6, IL-8) and anti-inflammatory cytokines (IL-10) (23-25), and the risk of postoperative complications was positively correlated with IL-6 and IL-8 (26).
CRP is a protein that rises sharply in plasma when the body is infected or damaged. It is a non-specific inflammatory marker and an important component of the body's non-specific immune mechanism, which can monitor the development of diseases. Surgical trauma leads to the production of a large number of cytokines, and IL-6 is the most important cytokine inducing hepatocytes to synthesize CRP (27,28). The larger the surgical trauma, the more obvious the inflammatory response, and the higher the serum CRP concentration (29,30).
In this study, by comparing CRP and IL-6 in the 2 groups before and after surgery, we found that CRP and IL-6 levels in the 2 groups at POD1 and POD3 were significantly higher than those before surgery (P<0.05). The values in Group CD increased more significantly than those in Group SD (P<0.05). This indicates that SLND induces a weaker inflammatory response and less non-specific immune damage to the body than systematic lymph node dissection.
Effects of SLND on cellular immunity
Cellular immunity refers to the immune response mediated by immune cells, particularly white blood cells, including lymphocytes and all kinds of phagocytic cells. Lymphocytes are a key component of the immune system and immune reactions. Changes in lymphocyte count can directly reflect the state of cellular immune function, and immunosuppression caused by lymph node dissection can be measured by the number of peripheral blood lymphocytes. Lymphocytes mainly include T cells, B cells, and NK cells, among which, T cells play an extremely important role in anti-tumor processes. Mature T cells express only CD4 or CD8, namely CD4+T cells or CD8+T cells. CD4+T cells play an active role in regulating immune responses, assisting with immunity, and directly killing tumor cells. CD8+T cells, on the other hand, are cytotoxic T cells, which have immunosuppressive effects in contrast to CD4+T cells (31). CD4+/CD8+ is an important indicator reflecting the body’s immune status and cellular immune activity. The smaller the ratio, the lower the immune response capacity, indicating that immune function is suppressed (32). Walsh et al. (33) pointed out that changes in T lymphocyte subsets could generally reflect the cellular immune status of patients after surgery or trauma.
NK cells recognize target cells as non-specific, and are an important immune factor with anti-tumor and anti-infection properties. Activated NK cells kill target cells such as virus-infected cells and tumors by releasing perforin, granular enzyme, and TNF-α, and express FasL. They can also exert immunomodulatory effects by secreting cytokines such as IFN-γ and TNF-α. Studies have shown that surgical trauma can reduce the number of T cells and NK cells in the blood (34). Compared with thoracotomy, minimally invasive surgery can increase the number of NK cells and T cells, which can better protect patients' postoperative immune function (35).
In this study, it was found that the proportions of lymphocytes and NK cells in Group SD and Group CD were significantly lower at POD1 and POD3 than those before surgery (P<0.05), but there was no significant difference between the 2 groups (P>0.05). The results showed that surgery could reduce lymphocytes and NK cells, but there were no significant differences in the effects of the 2 lymph node dissection methods on lymphocytes and NK cells. CD4+ cells and CD4+/CD8+ were significantly lower in Group CD than in Group SD at POD1 (P<0.05), and CD8+ cells were significantly lower in Group SD than in Group CD at POD3 (P<0.05). This indicates that SLND can more effectively mitigate the damage induced by surgery on cellular immune function, and can better alleviate the postoperative state of cellular immune suppression, so as to restore immune function more quickly.
Effects of SLND on humoral immunity
Humoral immunity refers to the specific immune response produced by the secretion of antibodies by plasma cells after the differentiation and proliferation of B cells and their transformation into plasma cells under the stimulation of antigens. Immunoglobulin (Ig) is an important effector molecule that mediates humoral immunity and can produce specific immune responses with corresponding antigens. The levels of IgG, IgA, and IgM can reflect the humoral immune function of the body.
IgG is the most abundant and most important Ig in serum and extracellular fluid, accounting for approximately 75–80% of total serum Ig. It activates complement in the immune response and neutralizes a variety of toxins. It also combines with NK cell and macrophage cell surface Fc receptors to play the role of conditioning and ADCC. There are 2 types of IgA: serotype and secretory type. Serotype IgA mainly exists in serum and accounts for 10–15% of total serum Ig, and can mediate the opsonophagocytosis of ADCC. Secretory type IgA is the main component of the body’s mucosal defense system. It is involved in local immunity of the mucosa and plays an important role as a barrier on the mucosa. It is the first line of defense against pathogens invading the body. IgM accounts for approximately 5–10% of total serum Ig. IgM is the earliest antibody in the first humoral immune response and the “leading force” against infection. Detection of IgM in serum indicates recent infection and is therefore used as a marker of recent infection. When the body is injured, the serum Ig concentration decreases, and the degree of decrease is closely related to the severity of the injury. Different surgical methods affect the postoperative serum specific Ig concentrations of patients, thus affecting patients’ humoral immune function (36).
In this study, it was found that IgG levels in Group SD and Group CD at POD1 and POD3 were significantly lower than those before surgery (P<0.05), but IgA and IgM levels were not significantly reduced (P>0.05). This indicates that both methods of lymph node dissection will damage humoral immunity mediated by IgG, but have less effect on humoral immunity mediated by IgA and IgM. There were no significant differences in IgG, IgA, and IgM levels between the 2 groups at the same point (P>0.05), indicating that there was no significant difference in humoral immunity between the 2 lymph node dissection methods.
In summary, minimally invasive thoracic surgery has become the main surgical method for thoracic diseases. Reducing the damage caused by lymph node dissection without metastasis is an important part of the development of minimally invasive thoracic surgery. By comparing SLND with systematic lymph node dissection, we further confirmed that SLND can better reduce operation times, intraoperative blood loss, pleural effusion drainage volume, the length of postoperative hospital stays, and the incidence of postoperative complications. At the same time, we also found that SLND can reduce the acute inflammatory response and mitigate the damage to cellular immune function caused by surgery, and alleviate postoperative cellular immunosuppression as soon as possible. There is no statistical difference in the effects of the 2 lymph node dissection methods on humoral immunity.
Similarly, this study has certain limitations, because the body’s immunity is affected by many factors (such as nutritional status, surgical methods, tumor staging, postoperative complications, etc.). Any change in any factor will lead to changes in the body’s immunity, which may require us to conduct more in-depth research.
Conclusions
Compared with systematic lymph node dissection, SLND has the following advantages: (I) it can reduce operation times, intraoperative blood loss, pleural effusion drainage volume, postoperative hospital-stay lengths, and the incidence of postoperative complications; (II) it can reduce the acute increase of cytokines (CRP, IL-6) caused by surgery and reduce the body’s acute inflammatory response and non-specific immune damage; (III) it can reduce the damage to cellular immune function caused by surgery, and can alleviate the state of cellular immunosuppression as soon as possible after surgery; (IV) there is no statistical difference in the effects of the 2 lymph node dissection methods on humoral immunity.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-524
Trial Protocol: Available at https://dx.doi.org/10.21037/tcr-21-524
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tcr-21-524
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-21-524). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of Linyi People’s Hospital (NO.: YX30066) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Verhagen AF, Bulten J, Shirango H, et al. The clinical value of lymphatic micrometastases in patients with non-small cell lung cancer. J Thorac Oncol 2010;5:1201-5. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Ishiguro F, Matsuo K, Fukui T, et al. Effect of selective lymph node dissection based on patterns of lobe-specific lymph node metastases on patient outcome in patients with resectable non-small cell lung cancer: a large-scale retrospective cohort study applying a propensity score. J Thorac Cardiovasc Surg 2010;139:1001-6. [Crossref] [PubMed]
- Schneider T, Hoffmann H, Dienemann H, et al. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J Thorac Oncol 2011;6:1162-8. [Crossref] [PubMed]
- Neeve SC, Robinson BW, Fear VS. The role and therapeutic implications of T cells in cancer of the lung. Clin Transl Immunology 2019;8:e1076. [Crossref] [PubMed]
- Zhang S, Pan SB, Lyu QH, et al. Postoperative Regulatory T-Cells and Natural Killer Cells in Stage I Nonsmall Cell Lung Cancer Underwent Video-assisted Thoracoscopic Lobectomy or Thoracotomy. Chin Med J 2015;128:1502-9. [Crossref] [PubMed]
- Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery 2000;127:117-26. [Crossref] [PubMed]
- Fu S, Qu PS, Cai SN. Effect of anesthetic methods on postoperative CD3+, CD4+ and CD4+CD25+ in patients with lung cancer undergoing radical operation. Oncol Lett 2018;16:6547-51. [Crossref] [PubMed]
- Zhao J, Jiang D, Du J, et al. Treatment of stage IIIA–N2 EGFR-mutant non-small cell lung adenocarcinoma. J Thorac Dis 2019;11:263-5. [Crossref] [PubMed]
- Pollock RE, Lotzova E, Stanford SD. Surgical stress impairs natural killer cell programming of tumor for lysis in patients with sarcomas and other solid tumors. Cancer 1992;70:2192-202. [Crossref] [PubMed]
- Christou NV, Meakins JL. Phagocytic and bactericidal functions of polymorphonuclear neutrophils from anergic surgical patients. Can J Surg 1982;25:444-8. [PubMed]
- Oberholzer A, Oberholzer C, Moldawerl LL. Sep sissyndromes: understanding the role of innate and acquired immunity. Shock 2001;16:83-96. [Crossref] [PubMed]
- Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555-72. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Ann Thorac Surg 1995;60:615-22. [Crossref] [PubMed]
- Zhong W, Yang XN, Bai JL, et al. Complete mediastinal lymphadenectomy: The core component of the multidisciplinary therapy in resectable non-small cell lung cancer. Eur J Cardiothorac Surg 2008;34:187-95. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg 2006;81:1028-32. [Crossref] [PubMed]
- Haruki T, Aokage K, Miyoshi T, et al. Mediastinal nodal involvement in patients with clinical stage I non-small-cell lung cancer: possibility of rational lymph node dissection. J Thorac Oncol 2015;10:930-6. [Crossref] [PubMed]
- Ohde Y, Nagai K, Yoshida J, et al. The proportion of consolidation to ground-glass opacity on high resolution CT is a good predictor for distinguishing the population of non-invasive peripheral adenocarcinoma. Lung Cancer 2003;42:303-10. [Crossref] [PubMed]
- Hashizume T, Yamada K, Okamoto N, et al. Prognostic significance of Thin-section CT scan findings in small-sized lung adenocarcinoma. Chest 2008;133:441-7. [Crossref] [PubMed]
- Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. [Crossref] [PubMed]
- Zhao JL, Guo HW, Yang P, et al. Selective lymph node dissection for clinical T1 stage non-small cell lung cancer. Transl Cancer Res 2019;8:2820-8. [Crossref]
- Wang G, Weng Y, Ishiguro Y, et al. The effect of tramadol on serum cytokine response in patients undergoing pulmonary lobectomy. J Clin Anesth 2005;17:444-50. [Crossref] [PubMed]
- Yim AP, Wan S, Lee TW, et al. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243-7. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Zhang LB, Wang B, Wang XY, et al. Influence of video assisted thoracoscopic lobectomy on immunological functions in non-small cell lung cancer patients. Med Oncol 2015;32:201. [Crossref] [PubMed]
- Wan S, LeClerc JL, Vincent JL. Cytokine responses to cardiopulmonary bypass: lessons learned from cardiac transplantation. Ann Thorac Surg 1997;63:269-76. [Crossref] [PubMed]
- Nijsten MW, Hack CE, Helle M, et al. Interleukin-6 and its telation to the humoral immune response and clinical parameters in burned patients. Surgery 1991;109:761-7. [PubMed]
- Ohzato H, Yoshizaki K, Nishimoto N, et al. Interleukin-6 as a new indicator of inflammatory status: Detection of serum levels of interleukin-6 and C-reactive protein after surgery. Surgery 1992;111:201-9. [PubMed]
- Leaver HA, Craig SR, Yap PL, et al. Lymphocyte responses following open and minimally invasive thoracic surgery. Eur J Clin Invest 2000;30:230-8. [Crossref] [PubMed]
- Craig SR, Leaver HA, Yap PL, et al. Acute phase responses following minimal access and conventional thoracic surgery. Eur J Cardiothorac Surg 2001;20:455-63. [Crossref] [PubMed]
- Smith R, Day A, Rockall T, et al. Advanced stereoscopic projection technology significantly improves novice performance of minimally invasive surgical skills. Surg Endosc 2012;26:1522-7. [Crossref] [PubMed]
- Handy JR Jr, Asaph JW, Douville EC, et al. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy. Eur J Cardiothorac Surg 2010;37:451-5. [PubMed]
- Walsh DS, Siritongtaworn P, Pattanapanyasat K, et al. Lymphocyte activation after non-thermal trauma. Br J Surg 2000;87:223-30. [Crossref] [PubMed]
- Xu P, Zhang P, Sun Z, et al. Surgical trauma induces postoperative T-cell dysfunction in lung cancer patients through the programmed death-1 pathway. Cancer Immunol Immunother 2015;64:1383-92. [Crossref] [PubMed]
- Ng CS, Lau KK. Surgical trauma and immune functional changes following major lung resection. Indian J Surg 2015;77:49-54. [Crossref] [PubMed]
- Chen L, Sun L, Lang Y, et al. Fast-track surgery improves postoperative clinical recovery and cellular and humoral immunity after esophagectomy for esophageal cancer. BMC Cancer 2016;16:449. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


