
Extraordinarily elevated CD33 expression in CD56+CD3– cells in the bone marrow of a patient with relapsed acute myeloid leukemia: a case report
Introduction
Based on genetic risk allogeneic stem cell transplantation (allo-SCT) is the only curative treatment for some forms of acute myeloid leukemia (AML), most patients still ultimately experience relapse (1,2). Knowledge of the mechanisms underlying treatment resistance and relapse remains limited. There are several potential mechanisms underlying immune evasion post allo-HSCT. For example, AML cells cannot be well recognized if there is impaired expression or genomic loss of human leukocyte antigen (HLA) (2). Upregulation of immune-checkpoint molecules, such as PD-1 can contribute to the AML immune escape. The production of some anti-inflammatory cytokines, such as interleukin-10 (IL-10) and transforming growth factor β (TGF-β) may also play a role in relapse. Moreover, anti-AML immune responses are lowered via reduced production of IL-15, which is important for NK cell activation (2,3).
Detection of minimal residual disease (MRD) by flow cytometry in patients with AML has been widely used to guide clinical management. The expression of CD33, a member of the sialic acid-binding immunoglobulin-like lectin family and assumed to be restricted to the myeloid lineage of immune cells, is generally used in MRD detection (4,5). Natural killer (NK) cells originate from hematopoietic stem cells (HSCs), primarily in the bone marrow, and play crucial roles in tumor surveillance and controlling tumor invasion (6,7). The NK cell function is finely tuned by activating and inhibitory receptors, allowing NK cells to discriminate between normal and aberrant cells (8). NK cells can kill AML directly via cytotoxicity and cytokines, such as IFN-γ, TNF-α, and CD107a. Therefore, it is important to maintain function of NK cells. A recent study reported that CD33 expression on NK cells could be a potential confounder for MRD detection, although the percentage of CD33+ NK cells was low (4). However, little is known about the interrelationships between extraordinarily high CD33 expression on CD56+CD3– cells and the impaired anti-tumor function of CD56+CD3– cells, as well as other immune characteristics in the bone marrow of patients with relapsed/refractory AML.
Here, we examined the characteristics and the impaired function of CD56+CD3– cells and demonstrated the abnormal proportion of CD33+ CD56+CD3– cells in the bone marrow of a patient with relapsed AML after allo-HSCT and remission induction, to ascertain whether abnormal CD56+CD3– cells are related to relapse.
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-733).
Case presentation
The case report was approved by the institutional review board of the First Affiliated Hospital of University of Science and Technology of China (2021-N(H)-120). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. Patient data were collected via retrospective chart review, which included clinical characteristics; comprehensive hematopathologic examination, such as Wright-Giemsa stain analyses, cytogenetic analysis and flow cytometry (9) (Appendix 1); and clinical outcome.
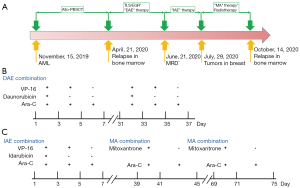
The characteristics of the 14-year-old girl were shown in (Table S1). The girl was diagnosed with AML-M1 in the First Affiliated Hospital of Anhui Medical University, in May 2019. Her karyotype was 46 XX, t(2; 17)(q31; q25), +8, t(16; 21)(p11; q22). PML/RARA and AML1/ETO fusion genes were not present. Then she received an allogeneic peripheral blood stem cell transplant (allo-PBSCT) from the matched sibling donor (MSD) in Children’s Hospital of Nanjing Medical University (15/November/2019). Relapse was confirmed in the bone marrow 6 months later. Thereafter, remission was induced using the DAE (daunorubicin, cytosine arabinoside, and etoposide) regimen once a month. This patient received daunorubicin (20 mg/m2/day) for 3 days (day 1–3), etoposide (VP-16, 120 mg/m2/day) for 5 days (day 1–5), and cytarabine (100 mg/m2/d) twice a day for 7 days (day 1–7). On 21/June/2020, MRD was found to be negative and the patient achieved complete remission (CR). The patient was then given the IAE (idarubicin, etoposide, and cytosine arabinoside) regimen: idarubicin (40 mg/m2/day) for 3 consecutive days, then etoposide (120 mg/m2/day) for 5 consecutive days, followed by cytarabine (100 mg/m2/d) twice a day for 7 consecutive days. On 29/July/2020, a secondary breast tumor was found. Thereafter, the patient was treated with the MA (mitoxantrone and cytosine arabinoside) regimen once a month for 2 months: mitoxantrone (10 mg/m2/day) for 3 consecutive days and cytarabine (100 mg/m2/d) twice a day for 7 consecutive days. During the MA regimen, the patient also received radiotherapy 12 times. Relapse was confirmed again on 14/October/2020 in bone marrow after the last radiotherapy with the treatment of anti-CD38 antibody (Figure 1).
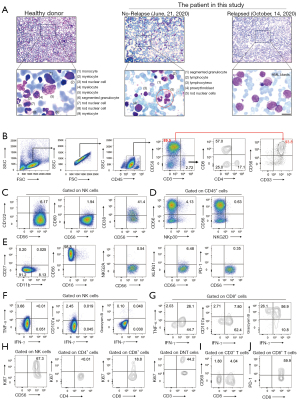
Through morphologic analysis of the leukemia cells in the bone marrow, we found that primitive cells accounted for 74% of the nucleated cells (Figure 2A). Thereafter, to investigate the immunological characteristics and the possible mechanism underlying the AML relapse, we isolated mononuclear cells from the bone marrow and systematically analyzed molecular expression related to NK cell function. The percentage of CD56+CD3– cells was nearly 90% when gated from CD45+ cells. Furthermore, CD33, generally assumed to be restricted to the myeloid lineage of immune cells [and not to erythrocytes, platelets, T cells, B cells, or NK cells (4)] was extraordinarily highly expressed on the CD56+CD3– cells (Figure 2B). The antibody used in this study were shown in (Table S2). As the activation of the CD56+CD3– cells was limited, we then analyzed CD69, CD38, NKG2D and NKp30 expression and found that their expressions were low (Figure 2C,2D). We characterized four populations of CD56+CD3– cells defined by CD11b and CD27, which represent the distinct stages of human NK cells from different tissues (10). The majority of CD56+CD3– cells had the CD11b-CD27- phenotype, which is reported to be an immature phenotype (11). Additionally, the CD56+CD3– cells had low expression of NKG2A, KLRG1, and PD-1 (Figure 2E). Moreover, human NK cells can be classified into two main classic subsets dependent on CD56 and CD16: CD56brightCD16- and CD56dimCD16+ NK cells (12). The former is considered efficient cytokine producers. The proportion of these cells was nearly 99% (Figure 2E). Then, to demonstrate the effector functions of the NK cells, we stimulated the mononuclear cells with PMA and ionomycin in the presence of monensin for 4 h. We found that the CD56+CD3– cells from the bone marrow exhibited an extremely low percentage (<0.1%) of polyfunctional effector IFN-γ+TNF-α+, IFN-γ+CD107a+, and IFN-γ+GranzymeB+ cells (Figure 2F). CD8+ T cells also play an important anti-tumor role, here we demonstrated that the proportion of polyfunctional effector IFN-γ+TNF-α+, IFN-γ+CD107a+ and IFN-γ+GranzymeB+ CD8+ T cells was normal (Figure 2G). This implied that the anti-tumor effect was heavily impaired in CD56+CD3– cells but not in CD8+ T cells. Furthermore, we demonstrated that Ki67 was highly expressed on these abnormal CD56+CD3– cells, but not on T cells (Figure 2H). Moreover, we verified the low expression of the early-activation marker CD69 on T cells (Figure 2I) and the high expression of PD-1 on CD8+ T cells (Figure 2J).
Discussion
Recently, Eckel et al. reported that CD33 expression on NK cells in AML was a potential confounder for MRD detection, and nearly 18% of MRD+ patients were CD33 positive. Furthermore, the CD33+ NK population accounted for a mean of 11.4% of NK cells (median 11.9%, range, 8.0–15.3%) (4). Here, we demonstrated that CD33 might not only be a potential confounder for MRD detection, but also a targetable molecule in our relapsed/refractory AML patient. We verified that the CD33+CD56+CD3– population accounted for 93.8% of the CD56+CD3– cells. Additionally, we found low expression of the activating receptors NKp30 and NKG2D. Importantly, we observed that CD56+CD3– cells had downregulated granzyme B, TNF-α, CD107a, and IFN-γ, suggesting impaired functionality. In addition, we speculated that these markers might be useful for predicting immune states in the tumor microenvironment. Given that several CD33-targeted therapies have been used in AML, and that they can mediate killing of AML blasts by CD56+CD3– cells (13-15), more attention should be paid to the influence of CD56+CD3– cells expressing CD33.
Taken together, these results showed that CD56+CD3– cells in the bone marrow of this patient with relapsed AML had abnormally high CD33 expression and severely impaired anti-tumor function, however, the immune function of CD8+ T cells in the bone marrow seemed to be normal. This study provides new perspectives on the mechanism underlying relapse after allo-SCT, and treatment with anti-CD33 monoclonal antibody may be worthy of attention.
Acknowledgments
The authors thank their colleagues at the First Affiliated Hospital of the University of Science and Technology of China for collaboration on the care of the patient. In addition, we appreciate the help from The Charlesworth Group, a high-quality publishing services company, for English language editing.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-733
Peer Review File: Available at https://dx.doi.org/10.21037/tcr-21-733
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-733). All authors report that this work was supported by China postdoctoral science foundation (2020M671910), and the Fundamental Research Funds for the Central Universities (WK9110000168, and WK9110000001). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The case report was approved by the institutional review board of the First Affiliated Hospital of University of Science and Technology of China (2021-N(H)-120). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bullinger L, Ehrich M, Döhner K, et al. Quantitative DNA methylation predicts survival in adult acute myeloid leukemia. Blood 2010;115:636-42. [Crossref] [PubMed]
- Christopher MJ, Petti AA, Rettig MP, et al. Immune Escape of Relapsed AML Cells after Allogeneic Transplantation. N Engl J Med 2018;379:2330-41. [Crossref] [PubMed]
- Carlsten M, Järås M. Natural Killer Cells in Myeloid Malignancies: Immune Surveillance, NK Cell Dysfunction, and Pharmacological Opportunities to Bolster the Endogenous NK Cells. Front Immunol 2019;10:2357. [Crossref] [PubMed]
- Eckel AM, Cherian S, Miller V, et al. CD33 expression on natural killer cells is a potential confounder for residual disease detection in acute myeloid leukemia by flow cytometry. Cytometry B Clin Cytom 2020;98:174-8. [Crossref] [PubMed]
- Freeman SD, Kelm S, Barber EK, et al. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood 1995;85:2005-12. [Crossref] [PubMed]
- Sinha C, Cunningham LC. An overview of the potential strategies for NK cell-based immunotherapy for acute myeloid leukemia. Pediatr Blood Cancer 2016;63:2078-85. [Crossref] [PubMed]
- Locatelli F, Pende D, Falco M, et al. NK Cells Mediate a Crucial Graft-versus-Leukemia Effect in Haploidentical-HSCT to Cure High-Risk Acute Leukemia. Trends Immunol 2018;39:577-90. [Crossref] [PubMed]
- Li H, Er Saw P, Song E. Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics. Cell Mol Immunol 2020;17:451-61. [Crossref] [PubMed]
- Zheng X, Qian Y, Fu B, et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol 2019;20:1656-67. [Crossref] [PubMed]
- Fu B, Zhou Y, Ni X, et al. Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity 2017;47:1100-1113.e6. [Crossref] [PubMed]
- Fu B, Li X, Sun R, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci U S A 2013;110:E231-40. [Crossref] [PubMed]
- Forconi CS, Cosgrove CP, Saikumar-Lakshmi P, et al. Poorly cytotoxic terminally differentiated CD56negCD16pos NK cells accumulate in Kenyan children with Burkitt lymphomas. Blood Adv 2018;2:1101-14. [Crossref] [PubMed]
- Sweeney C, Vyas P. The Graft-Versus-Leukemia Effect in AML. Front Oncol 2019;9:1217. [Crossref] [PubMed]
- Walter RB. Investigational CD33-targeted therapeutics for acute myeloid leukemia. Expert Opin Investig Drugs 2018;27:339-48. [Crossref] [PubMed]
- Jitschin R, Saul D, Braun M, et al. CD33/CD3-bispecific T-cell engaging (BiTE®) antibody construct targets monocytic AML myeloid-derived suppressor cells. J Immunother Cancer 2018;6:116. [Crossref] [PubMed]


