
The effects of ultrasound-guided radiofrequency ablation and laparoscopic hepatectomy in the treatment of small hepatocellular carcinoma: a retrospective analysis
Introduction
Hepatocellular carcinoma (HCC) ranks 6th among the most common cancers in the world and the 3rd cause of tumor related death in the world (1). China is the country with most liver cancer patients. It’s been reported that there are 854,000 new cases of HCC worldwide each year, and specifically in China there are 466,000 HCC patients, accounting for 55% of the world’s total HCC cases (2,3). Surgical resection is the main strategy for the treatment of HCC. Traditional open hepatectomy has disadvantages such as large intraoperative blood loss, more perioperative complications, and longer hospital stay (4). Laparoscopic hepatectomy (LH) is superior to open surgery in many regards (5). Previous studies (6,7) have shown that LH is safe, feasible and effective in treating HCC. Compared with open liver resection, laparoscopic liver resection can provide higher body surface aesthetics, less intraoperative blood transfusion, and shorter hospital stay. Previous studies have pointed out that radiofrequency ablation (RFA) therapy can also achieve a radical cure for liver tumors with a diameter of ≤6 cm, and its short-term and long-term survival rates are comparable to those of surgery. In addition, RFA has advantages independent of surgery with several advantages such as minimally invasive, shorter hospital stay, and higher patient satisfaction (8). Although both LH and RFA can achieve satisfactory results in the treatment of recurrent liver cancer, LH is prone to complications such as abdominal mucus, and RFA may have the problem of incomplete lesion removal (9). Therefore, there are no clear conclusions regarding the choice of recurrent HCC (RHCC) treatment. Previous studies have compared the efficacy of LH and RFA in the treatment of HCC, yet the sample size is small and the population is limited. The choice of treatment for HCC still lacks recommendations based on higher-level evidence-based medicine. Therefore, we aimed to compare the perioperative and long-term results of LH and RFA for the treatment of HCC, to provide evidence to the management of HCC.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-367).
Methods
Ethical concerns
This study was a retrospective design. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Sir Run Run Shaw Hospital (NO.: 161102-9c), and written informed consent was taken from all the patients.
Patients
HCC patients treated in our hospital from Aug 1, 2016 to Aug 31, 2018 were identified for potential candidate. All the patients either underwent LH or RFA treatment. The inclusion criteria were: (I) the single tumor had a diameter of ≤6 cm; (II) two or more examinations such as contrast-enhanced ultrasound, enhanced computed tomography (CT), enhanced magnetic resonance imaging (MRI) or puncture had been conducted for HCC diagnosis (10); (III) the patients was not in the decompensated stage of cirrhosis, and had no invasion of portal vein, hepatic arteriovenous or inferior vena cava, no metastasis to other organs outside the liver; (IV) patients either underwent LH or RFA treatment (V) patients were well informed and agreed to participant in this study. Exclusion criteria: (I) patients had ascites that was difficult to relieve; (II) patients with history of upper abdominal surgery; (III) patients did not agree to participant in this study. The patients randomly selected RFA or LH with their own willingness based on the criteria.
RFA treatment
The details of the RFA treatment were as follows: the radio frequency equipment chooses Talon radiofrequency needle (Valleylab Cooltip, USA) and related cold circulation system, the ultrasound contrast agent was sonovue (Bracco, Italy), and ultrasound contrast was routinely given before surgery to reconfirm the size, location and blood supply of the lesion, Peripheral organs and surrounding large blood vessels. We selected the puncture point and needle path, and punctured the radiofrequency needle into the tumor under ultrasound guidance. According to the size of the tumor and the surrounding conditions of the tumor, the power was generally 60–80 W, and the action duration was 10–20 min. For masses close to important organs and large blood vessels, ablation could be performed after injection of absolute alcohol on the adjacent sides of important organs or large blood vessels. In general, the treatment range should be fully covered by the tumor and more than 0.5 cm beyond the edge of the mass to eliminate the lesion. Liver contrast-enhanced ultrasound was performed immediately after ablation to observe the extent of liver tumor ablation and the boundary to the surrounding dangerous parts. If there was residual or suspicious residual, additional ablation could be performed. The needle was ablated while the needle was withdrawn to prevent bleeding or spread of the tumor.
LH treatment
LH was an anatomical hepatectomy in our study, and the details of the LH treatment were as following: after general anesthesia, the observation port under the umbilicus was taken to establish a pneumoperitoneum, a laparoscope was placed, and then other 3 to 4 trocars and related operating instruments were placed in the upper abdomen. We chose whether to place the hilar blocking band according to the specific situation. After the pre-cut line was drawn, the ultrasound knife gradually cut into the liver parenchyma. We carefully separated the tumor edge, the pulse triple system was separately ligated. And the cross-section adopted electrocoagulation or bipolar hemostasis. The liver cross-section was carefully checked with dry gauze, and absorbable hemostatic fibers (Johnson & Johnson, USA) was used for cover if necessary. After confirming that there was no obvious active bleeding and bile leakage, the abdominal drainage tube was placed.
Data collection
We observed and collected the characteristics of patients, including age, gender, cases of hepatitis B antigen positive and liver cirrhosis, alanine transaminase (ALT), aspartate transaminase (AST), blood platelet (PLT), American Society of Anesthesiologists (ASA) classification, alpha-fetoprotein level (AFP), Child-Pugh classification, fasting blood glucose. And we collected the treatment details including duration of surgery, estimated intraoperative blood loss, pain score on the first day after surgery, time to get out of bed after operation, time to oral eating, AST on the second day after surgery, C-reactive protein (CRP) on the second day after surgery, total medical cost. Furthermore, the related complications including abdominal infection, bleeding, biliary fistula and pleural effusion were detected and analyzed.
Postoperative follow-up
All the patients underwent 2-year long follow-up. All patients were rechecked with conventional B-ultrasound, enhanced CT or contrast-enhanced ultrasound and serum tumor markers in the outpatient clinic one month after surgery to determine whether the tumor was completely ablated or removed. Contrast-enhanced CT showed no enhancement in the ablation lesion, and contrast-enhanced ultrasound showed no contrast agent filling in the lesion, and it was judged that the tumor was completely ablated or removed. If the tumor was incompletely ablated, the patients would be re-admitted to the hospital for RFA. Routine B-ultrasound and serum tumor marker examinations was performed every 3–6 months. If routine B-ultrasound prompted suspicious lesions or serum tumor markers to rise again for a short period of time, further improve the enhanced CT, MRI or contrast-enhanced ultrasound examination to confirm the diagnosis. The deadline for follow-up was Aug 31, 2020 or if the patient died or was lost during the follow-up.
Statistical methods
We compared and analyzed the perioperative indicators and long-term results of the two groups of patients, and we used SPSS 22.0 statistical software for analysis. The continuous data were expressed as mean ± standard deviation, independent sample t-test was used for comparison between groups; χ2 test was used for categorical variables comparison. The Kaplan-Meier method was used to calculate the cumulative survival, and the survival curve was drawn by GraphPad PRISM 7.0 software. The test level was α=0.05, and P<0.05 indicated that the difference was statistically significant.
Results
The characteristics of patients
A total of 94 HCC patients were included, of which 46 patients underwent RFA treatment, and 48 patients underwent LH treatment. As presented in Table 1, there were not significant differences in the age, gender, cases of hepatitis B antigen positive and liver cirrhosis, number of HCC nodules, location of HCC, ALT, AST, PLT, ASA classification, AFP level, Child-Pugh classification, fasting blood glucose between two groups (all P>0.05), indicating that the preoperative characteristics of patients were comparable.
Table 1
| Variables | RFA group (n=46) | LH group (n=48) | χ2/t | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 56.33±12.49 | 57.24±13.23 | 12.154 | 0.238 |
| Male/female | 36/10 | 39/9 | 1.022 | 0.142 |
| Hepatitis B antigen positive, n (%) | 42 (91.30) | 43 (89.58) | 1.127 | 0.205 |
| liver cirrhosis, n (%) | 41 (89.13) | 40 (83.33) | 1.215 | 0.0189 |
| ALT (U/L), n (%) | 42.63±21.03 | 44.75±21.11 | 10.107 | 0.093 |
| AST (U/L), n (%) | 43.12±23.59 | 42.55±21.94 | 11.082 | 0.101 |
| PLT (×109/L), n (%) | 105.35±38.05 | 106.41±34.06 | 19.178 | 0.131 |
| Number of HCC nodules, mean ± SD | 3.18±1.96 | 3.11±1.93 | 1.244 | 0.076 |
| Location of HCC, n (%) | ||||
| Upper left | 7 (15.22) | 9 (18.75) | 2.472 | 0.101 |
| Lower left | 9 (19.57) | 8 (16.67) | ||
| Upper right | 16 (34.78) | 16 (33.33) | ||
| Lower right | 14 (30.43) | 15 (31.25) | ||
| ASA classification, n (%) | ||||
| I | 30 (65.22) | 32 (66.67) | 1.282 | 0.113 |
| II | 16 (34.78) | 16 (33.33) | ||
| AFP level, n (%) | ||||
| >400 ng/mL | 14 (30.43) | 13 (27.08) | 1.150 | 0.126 |
| ≤400 ng/mL | 32 (69.57) | 35 (72.92) | ||
| Child-Pugh classification, n (%) | ||||
| A | 44 (95.65) | 44 (91.67) | 1.082 | 0.094 |
| B | 2 (4.35) | 4 (8.33) | ||
| Fasting blood glucose (mmol/L), mean ± SD | 5.31±1.94 | 5.42±1.77 | 1.224 | 0.125 |
AFP, alpha-fetoprotein; ALT, alanine transaminase; ASA, American Society of Anesthesiologists; AST, aspartate transaminase; HCC, hepatocellular carcinoma; LH, laparoscopic hepatectomy; RFA, radiofrequency ablation; PLT, platelet; SD, standard deviation.
The intraoperative and postoperative variables comparison
As indicated in Table 2, the duration of surgery, estimated intraoperative blood loss, pain score on the first day after surgery, time to get out of bed after operation, time to oral eating, AST on the second day after surgery, CRP on the second day after surgery, total medical cost in RFA group were significantly less than that of LH group (all P<0.05).
Table 2
| Variables | RFA group (n=46) | LH group (n=48) | t | P |
|---|---|---|---|---|
| Duration of surgery (min) | 29.13±12.36 | 122.35±24.66 | 12.185 | 0.009 |
| Estimated intraoperative blood loss (mL) | 9.23±2.11 | 102.25±22.09 | 4.238 | 0.012 |
| Pain score on the first day after surgery | 0.92±0.34 | 3.11±0.77 | 1.123 | 0.014 |
| Time to get out of bed after operation (days) | 0.71±0.22 | 2.33±0.95 | 1.081 | 0.022 |
| Time to oral eating (days) | 1.12±0.14 | 2.43±0.61 | 1.124 | 0.048 |
| AST on the second day after surgery (U/L) | 76.33±36.75 | 154.95±54.21 | 19.130 | 0.015 |
| CRP on the second day after surgery (mg/L) | 28.17±12.92 | 33.73±20.06 | 12.036 | 0.035 |
| Total medical cost (RMB) | 22,740.12±2,093.26 | 31,042.84±3,452.81 | 112.729 | 0.011 |
AST, aspartate transaminase; CRP, C-reactive protein; LH, laparoscopic hepatectomy; RFA, radiofrequency ablation.
The postoperative complications comparisons between the two groups
As presented in Table 3, the incidence of abdominal infection and biliary fistula in RFA group were significantly less than that of LH group (all P<0.05), and there was no significant difference in the incidence of bleeding and pleural effusion between two groups (all P>0.05).
Table 3
| Variables | RFA group (n=46) | LH group (n=48) | χ2 | P |
|---|---|---|---|---|
| Abdominal infection | 2 (4.35) | 6 (12.50) | 1.024 | 0.014 |
| Bleeding | 1 (2.17) | 3 (6.25) | 1.116 | 0.069 |
| Biliary fistula | 1 (2.17) | 5 (10.42) | 1.094 | 0.012 |
| Pleural effusion | 1 (2.17) | 3 (6.25) | 1.116 | 0.069 |
LH, laparoscopic hepatectomy; RFA, radiofrequency ablation.
The survival prognosis of patients
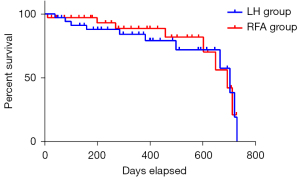
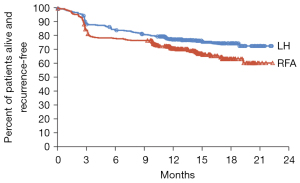
Of the 94 patients in this study, 8 patients were gradually lost to follow-up within 2 years, and the remaining 86 patients were completed for all the followed up. As showed in Figure 1, the 2-year overall survival of the two groups had no significant statistical difference (P=0.106). As showed in Figure 2, there was no significant difference in the recurrence-free survival between two groups (P=0.075).
Discussion
HCC currently ranks sixth in the world in incidence and third in tumor-related deaths (11). Although surgical resection is still the gold standard for radical treatment for HCC, radical resection is used for some small liver cancer patients. The overall survival rate at 3 years after surgery can be close to 90%, but the recurrence rate at 5 years after radical resection is as high as 70% (12,13). The LH technique has been used for nearly 30 years, and its safety, feasibility and effectiveness in the treatment of primary or recurrent liver cancer have been widely recognized (14). The efficacy of RFA in small liver cancer, especially with cancer nodules ≤3 cm, has been widely accepted by the medical profession and proved to be a treatment equivalent to surgical resection, and its 3-year overall survival rate is equivalent to that of open liver resection (15,16). The results of this present study have found that the RFA has more advantages over LH in reducing duration of surgery, estimated intraoperative blood loss, pain score on the first day after surgery, time to get out of bed after operation, time to oral eating, AST on the second day after surgery, CRP on the second day after surgery, total medical cost. Furthermore, the incidence of abdominal infection and biliary fistula in RFA groups are significantly less than that of LH group, and the 2-year overall survival of RFA and LH does not have significant difference, indicating that for the HCC with a diameter of ≤3 cm, RFA may be a better treatment option.
At present, for primary liver cancer with a diameter of ≤3 cm, the methods recognized at home and abroad that can achieve radical cure include liver transplantation, surgical resection, and RFA (17). Among them, liver transplantation has the best long-term effect, but due to its high requirements for technics, high cost, and lack of donors, its promotion and application are limited. With the improvement of laparoscopic equipment and technology, LH is being used more and more clinically. There are reports (18,19) showing that the short-term effect of LH for small liver cancer is better than that of open surgery, while the long-term effect is equivalent. The advantage of RFA is to cause coagulation and necrosis of tumor tissue, the treatment range is accurate, and the damage to normal liver tissue is greatly reduced (20). At the same time, it can activate the body’s immune function and can be applied to some patients with small tumors and tricky location that are difficult to undergo surgical treatment (21,22). Especially for primary liver cancer with a diameter of ≤3 cm, the effect is better, and patients with stage 0 liver cancer who cannot undergo liver transplantation in the BCLC staging treatment are recommended to give priority to ablation treatment by the guidelines (23,24).
Although LH is a minimally invasive treatment method compared with traditional open surgery, various factors such as the removal of part of the liver, the absorption of CO2 from the pneumoperitoneum during the operation, and the need for incisions in the abdominal wall to take specimens are still affecting the prognosis of patient (25-27), but these conditions are basically not present in RFA treatment. During our treatment, both groups of patients recovered smoothly, and no perioperative death occurred, indicating that both RFA and LH are generally safe and reliable. However, RFA has a lower complication rate in the treatment of HCC and has advantages in minimally invasiveness, which is consistent with previous reports (28,29).
According to the follow-up results, three cases of recurrence occurred after AFA. For cancers close to larger blood vessels, the heat loss during ablation caused poor ablation, but complete remission can still be achieved after re-ablation treatment (30). There was no residual tumor in the laparoscopic liver resection group, which may be related to the more thorough removal of tumor and surrounding liver tissue (31). At the same time, there was no significant difference in the 2-year overall survival between the two groups. However, the scope of application of the two methods may be different (32,33). For example, for a single small liver cancer located deep in the liver, surgical treatment is more difficult, and RFA may be applicable. While for small liver cancers like the caudal lobe of the liver, RFA is more difficult. LH may be more applicable at this time.
Several limitations must be considered in this present study. Firstly, since our study was a retrospective analysis, many details regarding the RFA and LH could not be included for analysis. Secondly, we selected HCC patients treated in our hospital from Aug 1, 2016 to Aug 31, 2018 as study population and made 2-year follow-up, the sample size was small, it might be not power enough to detect the differences between groups, future studies with larger sample size were needed. Thirdly, we only conduct 2-year long follow-up for HCC patients, it’s been reported that the recurrence rate of HCC was significantly increased 3 years after surgery, we will conduct longer follow-up to evaluate the effects and safety of RFA and LH in the future.
Conclusions
In conclusion, both RFA and LH are generally safe and effective for HCC treatment. RFA has many advantages of reduced perioperative complication, less trauma, and higher safety, and there is no significant difference in the 2-year survival prognosis. Clinicians can choose to carry out RFA or LH based on the patient’s condition and their own technical level to improve the prognosis of HCC patients.
Acknowledgments
Funding: This work was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-367
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tcr-21-367
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-367). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Sir Run Run Shaw Hospital (No.: 161102-9c), and written informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol 2020;72:250-61. [Crossref] [PubMed]
- Cheng S, Chen M, Cai J, et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus: 2016 edition. Oncotarget 2017;8:8867-76. [Crossref] [PubMed]
- Song P, Cai Y, Tang H, et al. The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines from 2001 to 2017. Biosci Trends 2017;11:389-98. [Crossref] [PubMed]
- Cui R, Yu J, Kuang M, et al. Microwave ablation versus other interventions for hepatocellular carcinoma: A systematic review and meta-analysis. J Cancer Res Ther 2020;16:379-86. [Crossref] [PubMed]
- Tan W, Deng Q, Lin S, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 2019;36:264-72. [Crossref] [PubMed]
- Jin S, Tan S, Peng W, et al. Radiofrequency ablation versus laparoscopic hepatectomy for treatment of hepatocellular carcinoma: a systematic review and meta-analysis. World J Surg Oncol 2020;18:199. [Crossref] [PubMed]
- Xiao N, Yu K, Yu S, et al. The paradigm of tumor shrinkage and rapid liver remnant hypertrophy for conversion of initially unresectable colorectal liver metastasis: a case report and literature review. World J Surg Oncol 2017;15:148. [Crossref] [PubMed]
- Aghayan DL, Kalinowski P, Kazaryan AM, et al. Laparoscopic liver resection for non-colorectal non-neuroendocrine metastases: perioperative and oncologic outcomes. World J Surg Oncol 2019;17:156. [Crossref] [PubMed]
- Pan YX, Long Q, Yi MJ, et al. Radiofrequency ablation versus laparoscopic hepatectomy for hepatocellular carcinoma: A real world single center study. Eur J Surg Oncol 2020;46:548-59. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Ayuso C, Rimola J, Vilana R, et al. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol 2018;101:72-81. [Crossref] [PubMed]
- Dimitroulis D, Damaskos C, Valsami S, et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol 2017;23:5282-94. [Crossref] [PubMed]
- Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019;157:54-64. [Crossref] [PubMed]
- Testino G, Leone S, Patussi V, et al. Hepatocellular carcinoma: diagnosis and proposal of treatment. Minerva Med 2016;107:413-26. [PubMed]
- Kim TH, Kim SY, Tang A, et al. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol 2019;25:245-63. [Crossref] [PubMed]
- Frenette CT, Isaacson AJ, Bargellini I, et al. A Practical Guideline for Hepatocellular Carcinoma Screening in Patients at Risk. Mayo Clin Proc Innov Qual Outcomes 2019;3:302-10. [Crossref] [PubMed]
- Liu PH, Hsu CY, Hsia CY, et al. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma ≤ 2 cm in a Propensity Score Model. Ann Surg 2016;263:538-45. [Crossref] [PubMed]
- Clark J, Mavroeidis VK, Lemmon B, et al. Intention to Treat Laparoscopic Versus Open Hemi-Hepatectomy: A Paired Case-Matched Comparison Study. Scand J Surg 2020;109:211-8. [Crossref] [PubMed]
- Yoh T, Cauchy F, Kawai T, et al. Laparoscopic right hepatectomy using the caudal approach is superior to open right hepatectomy with anterior approach and liver hanging maneuver: a comparison of short-term outcomes. Surg Endosc 2020;34:636-45. [Crossref] [PubMed]
- Li X, Wu YS, Chen D, et al. Laparoscopic hepatectomy versus radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Manag Res 2019;11:5711-24. [Crossref] [PubMed]
- Chong CC, Lee KF, Chu CM, et al. Laparoscopic Hepatectomy (with or without Robotic Assistance) versus Radiofrequency Ablation as a Minimally Invasive Treatment for Very Early-Stage or Early-Stage Hepatocellular Carcinoma. Dig Surg 2020;37:65-71. [Crossref] [PubMed]
- Takahashi H, Berber E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr 2020;9:49-58. [Crossref] [PubMed]
- Uhlig J, Sellers CM, Stein SM, et al. Radiofrequency ablation versus surgical resection of hepatocellular carcinoma: contemporary treatment trends and outcomes from the United States National Cancer Database. Eur Radiol 2019;29:2679-89. [Crossref] [PubMed]
- Kong J, Yao C, Ding X, et al. ATPase Inhibitory Factor 1 Promotes Hepatocellular Carcinoma Progression After Insufficient Radiofrequency Ablation, and Attenuates Cell Sensitivity to Sorafenib Therapy. Front Oncol 2020;10:1080. [Crossref] [PubMed]
- Lai C, Jin RA, Liang X, et al. Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma. J Zhejiang Univ Sci B 2016;17:236-46. [Crossref] [PubMed]
- Beppu T, Nitta H, Tsukamoto M, et al. Percutaneous radiofrequency ablation-assisted laparoscopic hepatectomy. Asian J Endosc Surg 2014;7:188-92. [Crossref] [PubMed]
- Weiss M, Mittermair C, Brunner E, et al. Inline radiofrequency pre-coagulation simplifies single-incision laparoscopic minor liver resection. J Hepatobiliary Pancreat Sci 2015;22:831-6. [Crossref] [PubMed]
- Yamashita YI, Imai K, Kaida T, et al. Multimodal radiofrequency ablation versus laparoscopic hepatic resection for the treatment of primary hepatocellular carcinoma within Milan criteria in severely cirrhotic patients: long-term favorable outcomes over 10 years. Surg Endosc 2019;33:46-51. [Crossref] [PubMed]
- Wilson GC, Geller DA. Evolving Surgical Options for Hepatocellular Carcinoma. Surg Oncol Clin N Am 2019;28:645-61. [Crossref] [PubMed]
- Tan HY, Gong JF, Yu F, et al. Long-Term Efficacy of Laparoscopic Radiofrequency Ablation in Early Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A 2019;29:770-9. [Crossref] [PubMed]
- Yao P, Gunasegaram A, Ladd LA, et al. InLine radiofrequency ablation-assisted laparoscopic liver resection: first experiment with stapling device. ANZ J Surg 2007;77:480-4. [Crossref] [PubMed]
- Qin L, Fei L. Use of Transthoracic Transdiaphragmatic Approach Assisted with Radiofrequency Ablation for Thoracoscopic Hepatectomy of Hepatic Tumor Located in Segment VIII. J Gastrointest Surg 2019;23:1547-8. [Crossref] [PubMed]
- Santambrogio R, Bruno S, Kluger MD, et al. Laparoscopic ablation therapies or hepatic resection in cirrhotic patients with small hepatocellular carcinoma. Dig Liver Dis 2016;48:189-96. [Crossref] [PubMed]


