
Patient outcomes after non-curative endoscopic submucosal dissection for early colorectal cancer: a single-center, retrospective cohort study
Introduction
Colorectal cancer (CRC) is a common malignant tumor of the digestive tract, as the third leading cause of cancer with increasing morbidity and mortality over recent decades worldwide (1). According to the latest global cancer data released by the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO), approximately 1.93 million individuals were diagnosed with CRC in 2020, of which 935,173 died from the disease; 555,477 cases and 286,162 deaths were recorded for individuals in China (2). Treatment strategies for CRC are based on TNM tumor stage, and include endoscopic treatment, surgery, chemotherapy, radiotherapy and other biological immunological treatments (3). The prognosis of CRC is highly dependent on the early detection and accurate staging. The 5-year survival rate of metastatic CRC was less than 14%, while that of early CRC was more than 90% (1,4).
Endoscopic resection is a minimally invasive therapeutic modality for early CRC. Currently, the most common endoscopic techniques available for the removal of colon polyps are polypectomy, endoscopic mucosal resection (EMR), and endoscopic submucosal dissection (ESD). ESD is endorsed for lesions that have a high likelihood of cancer invading the superficial submucosa and for polyps that cannot be removed by EMR due to fibrosis in the submucosal space or post-EMR recurrences (5). Previously, surgical resection was the standard treatment for all early cases of CRC; however, endoscopic resection is now a first-line treatment for early CRC without regional lymph node (LN) metastasis. With the recent advances of endoscopic technology, ESD has become the primary therapeutic approach for early CRC, because it requires shorter hospital stays, causes fewer adverse events, does not require bowel resection, and is more economical than surgical resection (6). However, when the pathological evaluation of specimens after ESD shows particular signs, such as submucosal invasion greater than 1,000 μm, positive resection margin, poor differentiation, lympho-vascular infiltration, perineuronal invasion, and tumor budding, it is regarded as non-curative dissection, and additional surgery with lymphadenectomy is recommended owing to the high-risk factors for residual cancer or LN metastasis (7). Moreover, in clinical practice, some patients who receive non-curative ESD for early CRC refuse additional surgical intervention on account of advanced age or preservation of the anal sphincter. On the other hand, few patients are found to have either residual cancer or LN metastasis after this additional surgery.
Currently, research on non-curative ESD for early CRC mostly focuses on pathological features predicting risk of residual cancer or LN metastasis (7-10). Very few studies have been found to concentrate on the treatment patterns and outcomes. In this study, we retrospectively reviewed disease course in 180 Chinese patients with early-stage CRC to investigate the treatment patterns and outcomes in patients after non-curative ESD for this type of cancer. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tcr-21-1545).
Methods
Patient cohort
All consecutive patients who received non-curative ESD for early CRC in the Cancer Hospital at the Chinese Academy of Medical Sciences from December 2010 to December 2019 were included in this retrospective study. The inclusion criteria in our study were as follows, based on the Japanese Society for Cancer of the Colon and Rectum (11): (I) horizontal or vertical positive resection margin; (II) submucosal invasion greater than 1,000 μm; (III) poor differentiation; (IV) lympho-vascular infiltration; (V) perineuronal invasion; (VI) tumor budding. Patients were excluded from the study if they: (I) had received colorectal surgery before ESD; (II) did not have complete medical and histopathology records; (III) did not have available follow-up data.
Ultimately, a total of 180 patients were included in the analysis. Patients were classified into two groups according to the treatment method: those who received additional surgery (“additional surgery”), and those who did not receive additional surgery, but were kept under surveillance (“surveillance-only”).
The study was approved by the Institutional Review Board Committee of the Cancer Hospital, Chinese Academy of Medical Sciences (approval No.18-015/1617) and individual consent for this retrospective analysis was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Histopathological examination
After fixation with formalin and processing, all endoscopically-resected specimens were successively cut into parallel 3–4-mm- and 2-mm-thick sections, respectively. The examination of early CRC pathological features was performed using three methods: hematoxylin and eosin (H&E) staining for identifying perineuronal invasion; D2-40 immunostaining for lymphatic invasion; and Victoria blue staining for venous invasion. Lymphatic invasion was defined as the invasion of at least one tumor cell into a lymphatic vessel in the D2-40 stained sections. Venous invasion was defined as a tumor deposit in a space surrounded by a rim of smooth muscle and/or containing red blood corpuscles. Budding was defined as the presence of cancer cell clusters made up of between one and four constituent cells, present at the stroma of the invasive front, as evaluated in H&E-stained sections. Standard histopathological evaluations were performed by at least two independent, experienced gastrointestinal pathologists to assess resection margin status and histological characteristics. In addition to the gross appearance of lesions (including location, size, shape, with or without ulceration and mucosal lesions), detailed and standardized pathology reports including the tumor pathological tissue types, resection margin status, tumor infiltration depth, and presence of vascular involvement were produced.
Treatment of early CRC
ESD enables en bloc resection of early CRC without LN metastasis, resulting in very low rates of local recurrence, high-quality pathologic specimens for accurate histopathologic diagnosis, and potentially curative treatment of early adenocarcinoma without resorting to major surgical resection (12). In the ESD procedures carried out in this study, a mixture of 4% hyaluronic acid and normal saline with a small amount of indigo carmine and epinephrine (0.001 mg/mL) was injected into the submucosal layer. After lifting up the lesion, a mucosal incision followed by submucosal dissection was performed using knives. Specimens were then fixed using pins in formalin solution and examined histologically by two independent pathologists.
When curative criteria were not met, additional surgery was recommended. However, after non-curative ESD for early CRC, some patients did not undergo additional surgery, on account of advanced age or preservation of the anal sphincter. Operations were performed by experienced surgeons according to the total mesorectal excision or complete mesenteric resection principles.
Follow-up and clinical outcomes
Follow-up information was retrieved from outpatient follow-up review or telephone follow-up records. Colonoscopy was conducted at 3, 6, and 12 months after ESD, and annually thereafter. Serum carcinoembryonic antigen, chest X-ray, and abdominopelvic computed tomography were carried out at 6-month intervals for 3 years and then annually thereafter, to determine local recurrence and distant metastasis. The endpoint of follow-up was January 2021. Clinical outcomes included overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS).
Statistical analysis
Quantitative variables were reported as mean ± standard deviation (SD) or interquartile range (IQR), as appropriate. Categorical variables were reported as number (frequency); chi-square or Fisher’s exact tests were used for comparisons, as appropriate. The association between treatment method and patient clinicopathologic characteristics was evaluated using chi-squared or Fisher’s exact tests, as appropriate. Median follow up was calculated using the reverse Kaplan-Meier method. The associations between treatment method and clinical outcomes were evaluated using the Kaplan-Meier method with log-rank test. Two-sided P values of <0.05 were considered statistically significant. All statistical analyses were performed using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 180 patients were enrolled in this retrospective study: 85 patients (47.2%) were included in the “additional surgery” group and 95 patients (52.8%) in the “surveillance-only” group. The mean age of the patients was 59.8±10.1 years old.
No significant differences were observed in gender, tumor size, resection margin, vertical margin, lateral margin, lympho-vascular invasion or tumor budding between two groups. Compared with those in the additional surgery group, patients in the surveillance-only group tended to be older (61.5±10.0 versus 58.0±9.8; P=0.029). Furthermore, tumors in the surveillance-only group were located significantly more often in the rectum (73.7% versus 55.3%; P=0.029), poorly differentiated less often (10.5% versus 25.9%; P=0.007), with a shallower depth of invasion (2,465.0±2,187.9 versus 3,992.7±2,881.6; P=0.002), and less perineuronal invasion (0.0% versus 4.7%; P=0.048) than in the surveillance-only group; fewer high-risk factors for residual cancer or LN metastasis (P=0.0003) were observed in the surveillance-only group compared with those in the additional surgery group. Patients’ demographic and clinicopathologic characteristics are summarized in Table 1.
Table 1
| Variable | Total (n=180) | Surveillance-only group (n=95) | Additional surgery group (n=85) | P value |
|---|---|---|---|---|
| Gender, n (%) | 0.195 | |||
| Male | 101 (56.1) | 49 (51.6) | 52 (61.2) | |
| Female | 79 (43.9) | 46 (48.4) | 33 (38.8) | |
| Age at diagnosis, years | 59.8±10.1 | 61.5±10.0 | 58.0±9.8 | 0.029 |
| <60 | 82 (45.6) | 36 (37.9) | 46 (54.1) | |
| ≥60 | 98 (54.4) | 59 (62.1) | 39 (45.9) | |
| Tumor location, n (%) | 0.029 | |||
| Rectum | 117 (65.0) | 70 (73.7) | 47 (55.3) | |
| Left colon | 48 (26.7) | 18 (18.9) | 30 (35.3) | |
| Right colon | 15 (8.3) | 7 (7.4) | 8 (9.4) | |
| Tumor size, cm | 2.6±1.1 | 2.5±0.9 | 2.7±1.2 | 0.062 |
| <2 | 59 (32.8) | 37 (38.9) | 22 (25.9) | |
| ≥2 | 121 (67.2) | 58 (61.1) | 63 (74.1) | |
| Resection margin, n (%) | 0.797 | |||
| Negative | 85 (47.2) | 44 (46.3) | 41 (48.2) | |
| Positive | 95 (52.8) | 51 (53.7) | 44 (51.8) | |
| Vertical margin, n (%) | 0.881 | |||
| Negative | 90 (50.0) | 48 (50.5) | 42 (49.4) | |
| Positive | 90 (50.0) | 47 (49.5) | 43 (50.6) | |
| Lateral margin, n (%) | 0.724 | |||
| Negative | 172 (95.6) | 90 (94.7) | 82 (96.5) | |
| Positive | 8 (4.4) | 5 (5.3) | 3 (3.5) | |
| Histology, n (%) | 0.007 | |||
| Differentiateda | 148 (82.2) | 85 (89.5) | 63 (74.1) | |
| Undifferentiatedb | 32 (17.8) | 10 (10.5) | 22 (25.9) | |
| Depth of invasion, μm | 3,164.8±2,633.9 | 2,465.0±2,187.9 | 3,992.7±2,881.6 | 0.002 |
| <1,000 | 42 (23.3) | 31 (32.6) | 11 (12.9) | |
| ≥1,000 | 138 (76.7) | 64 (67.4) | 74 (87.1) | |
| Lympho-vascular invasion, n (%) | 0.187 | |||
| Negative | 147 (81.7) | 81 (85.3) | 66 (77.6) | |
| Positive | 33 (18.3) | 14 (14.7) | 19 (22.4) | |
| Perineuronal invasion, n (%) | 0.048 | |||
| Negative | 176 (97.8) | 95 (100.0) | 81 (95.3) | |
| Positive | 4 (2.2) | 0 (0.0) | 4 (4.7) | |
| Tumor budding, n (%) | 0.311 | |||
| Negative | 94 (52.2) | 53 (58.8) | 41 (48.2) | |
| Positive | 86 (47.8) | 42 (44.2) | 44 (51.8) | |
| Number of high-risk factors*, n (%) | 0.0003 | |||
| 1 | 55 (30.6) | 41 (43.2) | 14 (16.5) | |
| 2 | 60 (33.3) | 29 (30.5) | 31 (36.5) | |
| ≥3 | 65 (36.1) | 25 (26.3) | 40 (47.0) | |
| Duration (months)c | – | 52.2±26.5 | 1.5±1.8 | – |
a, well/moderately differentiated or high-grade intraepithelial neoplasia containing malignant components; b, poorly differentiated adenocarcinoma and signet-ring cell adenocarcinoma; c, duration is the period of the observation after endoscopic submucosal dissection; *, high-risk factors for residual cancer or lymph node (LN) metastasis include: (I) horizontal or vertical positive resection margin; (II) submucosal invasion greater than 1,000 μm; (III) poor differentiation; (IV) lymphovascular infiltration; (V) perineuronal invasion; (VI) tumor budding.
Clinical outcomes
The median follow-up time of all patients in this study was 42 months [95% confidence interval (CI): 37.4–46.6]. There was no significant differences in 5-year OS (92.6% versus 92.7%, P=0.355), 5-year DFS (94.7% versus 91.9%, P=0.340) and 5-year CSS (93.8% versus 92.7%, P=0.791) between the additional surgery and surveillance-only groups, respectively. Total recurrence rates were 4.7% and 9.5% (P=0.217) in the additional surgery group and surveillance-only group, respectively (Table 2, Figures 1-3).
Table 2
| Items | Additional surgery group (n=85) | Surveillance-only group (n=95) | P value |
|---|---|---|---|
| Median duration of follow-up (months), (95% CI) | 37.0 (33.6–40.4) | 46.0 (28.3–63.7) | – |
| 5-year overall survival, % | 92.6% | 92.7% | 0.355 |
| 5-year disease-free survival, % | 94.7% | 91.9% | 0.340 |
| 5-year cancer-specific survival, % | 93.8% | 92.7% | 0.791 |
| No recurrence, n (%) | 81 (95.3) | 86 (90.5) | 0.217 |
| Total recurrence, n (%) | 4 (4.7) | 9 (9.5) | |
| Local recurrence only, n (%) | 1 (1.2) | 3 (3.2) | – |
| Distant/LN metastasis only, n (%) | 0 (0.0) | 2 (2.1) | – |
| Both local and distant metastases, n (%) | 3 (3.5) | 4 (4.2) | – |
CI, confidence interval; LN, lymph node.
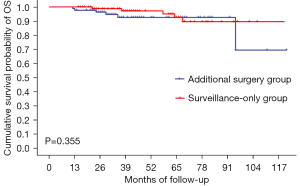
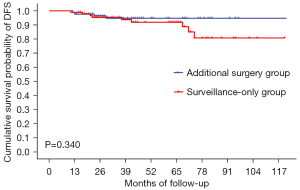

Local recurrence or distant/LN metastasis was observed in 13 patients (4 in the additional surgery group and 9 in the surveillance-only group). In the additional surgery group, four patients had local recurrence, three of whom had distant metastasis (one LN metastasis, one LN and bone metastasis, and one LN and liver metastasis). All four patients died from disease recurrence, had deep submucosal invasion (≥3,000 μm) and had at least three high-risks factors for residual cancer and LN metastasis. In the surveillance-only group, three patients had local recurrence only, two had distant/LN metastasis only, and four had both local and distant metastasis. Five of these patients died from disease recurrence. Among the patients whose submucosal invasion depth was measured, the value was <1,000 μm in only one patient, in whom tumor budding was also found. The characteristics of patients with disease recurrence after treatment are presented in Table 3.
Table 3
| No. of cases | Gender | Age at diagnosis | Tumor location | Resection margin | Histology | Depth of invasion (μm) | Lymphovascular invasion | Perineuronal invasion | Tumor budding | Number of risks | Recurrence site | Time to recurrence (months) | Alive/ dead |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The additional surgery group | |||||||||||||
| 1 | Male | 63 | Right colon | (−) | Undifferentiated | 3,050 | (+) | (−) | (−) | 3 | Local, LN, bone | 23 | Dead |
| 2 | Male | 74 | Rectum | (+) | Undifferentiated | 5,000 | (+) | (−) | (−) | 4 | Local | 13 | Dead |
| 3 | Male | 60 | Left colon | (+) | Differentiated | 3,000 | (+) | (−) | (−) | 3 | Local, LN | 12 | Dead |
| 4 | Male | 54 | Rectum | (−) | Undifferentiated | 3,500 | (+) | (−) | (−) | 3 | Local, LN, liver | 29 | Dead |
| The surveillance-only group | |||||||||||||
| 1 | Male | 64 | Rectum | (−) | Differentiated | 4,250 | (−) | (−) | (−) | 1 | Local, LN | 30 | Dead |
| 2 | Male | 61 | Left colon | (−) | Undifferentiated | 1,500 | (−) | (−) | (−) | 2 | Local | 11 | Alive |
| 3 | Male | 57 | Rectum | (+) | Undifferentiated | 1,300 | (−) | (−) | (−) | 3 | Local | 74 | Alive |
| 4 | Male | 64 | Left colon | (−) | Differentiated | 2,050 | (−) | (−) | (−) | 1 | Local, LN, liver | 21 | Dead |
| 5 | Male | 62 | Right colon | (+) | Differentiated | 150 | (−) | (−) | (+) | 2 | Local, LN | 22 | Dead |
| 6 | Female | 60 | Left colon | (+) | Differentiated | 1,000 | (−) | (−) | (−) | 2 | Local | 71 | Alive |
| 7 | Female | 52 | Rectum | (+) | Differentiated | 2,000 | (−) | (−) | (+) | 3 | Lung | 68 | Dead |
| 8 | Male | 70 | Rectum | (−) | Differentiated | 3,500 | (−) | (−) | (−) | 1 | Local, LN | 37 | Dead |
| 9 | Male | 61 | Rectum | (+) | Differentiated | 1,000 | (−) | (−) | (+) | 3 | LN | 30 | Alive |
−, negative; +, positive. LN, lymph node.
Discussion
ESD is a minimally-invasive treatment that has been widely applied in recent years for the treatment of early-stage CRC; however, both patients and doctors have concerns about the possibility of disease recurrence due to non-curative ESD (13). Several studies have reported histopathologic and other risk factors for residual cancer and LN metastasis after non-curative endoscopic resection of early-stage CRC (7,9,10). Despite the high clinical and practical significance, until now there have been few reports on outcomes from ESD in early-stage CRC (14-16).
To our knowledge, this is the first retrospective cohort study in China comparing clinical outcomes from ESD alone versus ESD with additional surgery. Eun Young Park and colleagues concluded that the surveillance-only approach could be considered as an alternative option for early-stage CRC in select patients undergoing non-curative ESD (16). They calculated 5-year OS as 75.3% and 92.6% in surveillance-only and additional surgery groups, respectively, but the hazard ratio (HR) for additional surgery versus surveillance-only in non-curative ESD was not statistically significant. However, they did not compare clinicopathologic characteristics between the two groups. In our study, compared with those in the additional surgery group, patients in the surveillance-only group tended to be older; their tumors were significantly more often located in the rectum, which were better differentiated with a shallower depth of invasion and less perineuronal invasion compared with those in the additional surgery group.
The investigation of treatment patterns associated with additional treatment after non-curative ESD for early-stage CRC patients, with or without additional surgery, is a highlight of our study. Over a median follow-up time of 42 months, there was no significant difference in 5-year OS, 5-year DFS and 5-year CSS between the additional surgery and surveillance-only groups. Similar results have been reported previously. A review of early-stage CRC patients in the SEER (Surveillance, Epidemiology and End Results) database showed similar risks of death between additional surgery and surveillance-only groups, after accounting for age and comorbidities and adjusting for propensity quintile (17); this is consistent with our results. In the reports by Yamashita and colleagues and Eun Young Park and colleagues, differences in the 5-year OS rates in additional surgery and surveillance-only groups in patients who received non-curative ESD were not statistically significant (16,18). Surveillance or close follow-up after non-curative ESD for early CRC may serve as good alternatives to additional surgery, especially in patients with more advanced ages or high anesthetic- risks. Individual patient choice should be considered in addition to the clinical opinion of their physician to determine the best approach to treatment; therefore, further high-quality cohort studies with long follow-up periods should be conducted to confirm the benefits of additional surgery and surveillance following non-curative ESD.
Overall, recurrence rates between 0 and 16.2% have been reported for early-stage CRC after ESD (14-16,18,19). Yoda and colleagues (14) reported that among patients with high-risk features, recurrence rates were 3.6% and 6.6% in the additional surgery and surveillance-only groups, respectively. Ikematsu and colleagues (15) reported that the disease recurrence rate in low-risk patients undergoing only endoscopic resection for submucosal colon and rectal cancer was 0% versus 6.3%,respectively. In high-risk patients undergoing endoscopic resection only, and those undergoing surgical resection that included LN dissection, these values were 1.4% versus 16.2%, and 1.9% versus 4.5%, respectively. Asayama and colleagues (19) found that disease recurrence rates in patients who underwent surgical resection alone, additional surgery after ESD, and ESD alone were 4.3%, 6.6%, and 4.4%, respectively. In our study, the recurrence rates were 4.7% (4/85) and 9.5% (9/95) in the additional surgery and surveillance-only groups, respectively. These findings suggest that, from a disease recurrence perspective, additional surgery is warranted in the early-stage CRC in the cases of non-curative ESD. However, various situations need to be taken into account to determine the best treatment pattern for patients after non-curative ESD for early CRC, and comprehensive treatment decision should be made based on other factors, such as age and significant comorbidities.
This study has some limitations. First, this study was a single-center retrospective cohort study, along with selection bias. However, it would not be ethically sound to conduct a randomized study to prospectively compare clinical outcomes in similar surveillance-only vs. additional surgery groups. Second, statistical power in this study was likely not sufficient to discern small differences between groups in the comprehensive pathologic factors that were measured. Therefore, large-scale multicenter cohort studies are needed to assess long-term outcomes after treatment in early-stage CRC under current practice.
Conclusions
ESD results in favorable outcomes for patients with early CRC. Surveillance in patients who receive non-curative ESD may be an alternative option for those with advanced age and fewer high-risk factors for residual cancer or LN metastasis.
Acknowledgments
We sincerely thank all the reviewers and editors for their supportive suggestions.
Funding: This work was supported by National Key R&D Program of China (No. 2017YFC0908203), and Science and Technology Project of Chaoyang District, Beijing (No. CYSF-1931).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tcr-21-1545
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tcr-21-1545
Peer Review File: Available at https://dx.doi.org/10.21037/tcr-21-1545
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-1545). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board Committee of the Cancer Hospital, Chinese Academy of Medical Sciences (approval No. 18-015/1617) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Latest global cancer data: Cancer burden rises to 19.3 million new cases and 10.0 million cancer deaths in 2020: International Agency for Research on Cancer; 2020. Available online: https://www.iarc.fr/fr/news-events/latest-global-cancer-data-cancer-burden-rises-to-19-3-million-new-cases-and-10-0-million-cancer-deaths-in-2020/
- Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers 2015;1:15065. [Crossref] [PubMed]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- Yang D, Othman M, Draganov PV. Endoscopic Mucosal Resection vs Endoscopic Submucosal Dissection For Barrett's Esophagus and Colorectal Neoplasia. Clin Gastroenterol Hepatol 2019;17:1019-28. [Crossref] [PubMed]
- Park CH, Yang DH, Kim JW, et al. Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer. Clin Endosc 2020;53:142-66. [Crossref] [PubMed]
- Cheng P, Lu Z, Zhang M, et al. Is Additional Surgery Necessary After Non-Curative Endoscopic Submucosal Dissection for Early Colorectal Cancer? J Invest Surg 2021;34:889-94. [Crossref] [PubMed]
- Makimoto S, Takami T, Hatano K, et al. Additional surgery after endoscopic submucosal dissection for colorectal cancer: a review of 53 cases. Int J Colorectal Dis 2019;34:1723-9. [Crossref] [PubMed]
- Chen T, Zhang YQ, Chen WF, et al. Efficacy and safety of additional surgery after non-curative endoscopic submucosal dissection for early colorectal cancer. BMC Gastroenterol 2017;17:134. [Crossref] [PubMed]
- Kim KM, Eo SJ, Shim SG, et al. Risk factors for residual cancer and lymph node metastasis after noncurative endoscopic resection of early colorectal cancer. Dis Colon Rectum 2013;56:35-42. [Crossref] [PubMed]
- Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 2018;23:1-34. [Crossref] [PubMed]
- Emmanuel A, Gulati S, Burt M, et al. Colorectal endoscopic submucosal dissection: patient selection and special considerations. Clin Exp Gastroenterol 2017;10:121-31. [Crossref] [PubMed]
- Dang H, de Vos Tot Nederveen Cappel WH, van der Zwaan SMS, et al. Quality of life and fear of cancer recurrence in T1 colorectal cancer patients treated with endoscopic or surgical tumor resection. Gastrointest Endosc 2019;89:533-44. [Crossref] [PubMed]
- Yoda Y, Ikematsu H, Matsuda T, et al. A large-scale multicenter study of long-term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy 2013;45:718-24. [Crossref] [PubMed]
- Ikematsu H, Yoda Y, Matsuda T, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology 2013;144:551-9; quiz e14. [Crossref] [PubMed]
- Park EY, Baek DH, Lee MW, et al. Long-Term Outcomes of T1 Colorectal Cancer after Endoscopic Resection. J Clin Med 2020;9:2451. [Crossref] [PubMed]
- Bhangu A, Brown G, Nicholls RJ, et al. Survival outcome of local excision versus radical resection of colon or rectal carcinoma: a Surveillance, Epidemiology, and End Results (SEER) population-based study. Ann Surg 2013;258:563-9; discussion 569-71. [Crossref] [PubMed]
- Yamashita K, Oka S, Tanaka S, et al. Long-term prognosis after treatment for T1 carcinoma of laterally spreading tumors: a multicenter retrospective study. Int J Colorectal Dis 2019;34:481-90. [Crossref] [PubMed]
- Asayama N, Oka S, Tanaka S, et al. Long-term outcomes after treatment for T1 colorectal carcinoma. Int J Colorectal Dis 2016;31:571-8. [Crossref] [PubMed]


