
Magnetic resonance imaging of pancreatic malignancy
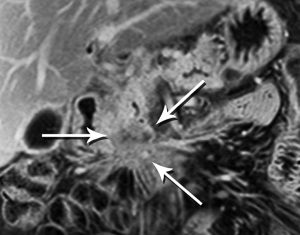
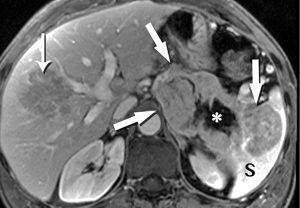
Pancreatic malignancies continue to present a huge challenge not only to the surgeon, the radiation oncologist, and the medical oncologist, but to the radiologist as well. The pancreas is centered in the abdomen, tucked into the duodenal sweep, behind both stomach and transverse colon, with associated image degradation from bowel gas and motion. To complicate the picture further, nearly all the important vessels in the abdomen, along with the bile duct, are in intimate association with the pancreas. Even without these anatomic factors, visualization of pancreatic ductal adenocarcinoma (PDA) would be difficult due to the tumor’s generally infiltrative nature, poor margination, and relatively bland enhancement pattern (Figure 1). Pancreatic duct obstruction is the rule, generally resulting in congested, inflamed background pancreatic parenchyma, another feature obscuring the actual tumor and its margins (Figure 2). Detection and margination of a pancreatic tumor can be problematic for any imaging modality, largely because of the hypovascular and infiltrative characteristics mentioned above, and because of mimics like focal IgG4-related pancreatitis, sequelae of trauma or pancreatitis, groove pancreatitis, metastases to the pancreas, and intrapancreatic splenule. Further, since the pancreas lacks a capsule, tumor rapidly infiltrates into the peripancreatic tissues and encases critical vessels (Figure 3). Given these challenges for detection and staging, it is not surprising that as many as 57% of laparotomies performed with curative intent discover locally advanced PDA or metastatic disease unrecognized by the preoperative workup (1).
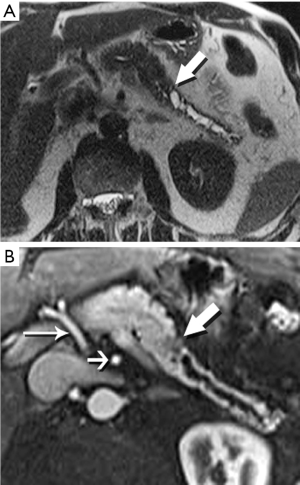
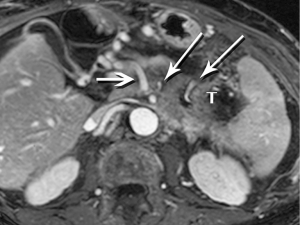
The radiologist is tasked not only with detecting a pancreatic tumor, but with determining whether it is currently, or potentially, surgically resectable. This is largely dependent on a tumor’s relationship with local arteries, specifically the celiac trunk, hepatic artery, and superior mesenteric artery (SMA). Attention must also be given to the status of the inferior vena cava, portal vein (PV), superior mesenteric vein (SMV), and first jejunal artery and vein. Current staging standards, published and periodically updated by the National Comprehensive Cancer Network (NCCN), describe three categories: resectable, borderline resectable, and unresectable, which are largely determined by these relationships, with slight variation depending on where in the pancreas the tumor resides (Figure 4). For example, a pancreatic head tumor is considered to have borderline resectability if it contacts the SMA, but the abutment must be less than 180 degrees (2).
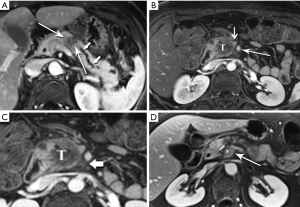
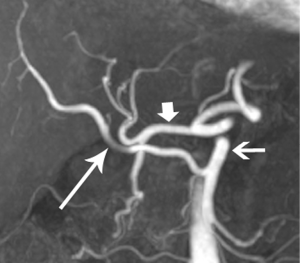
In our practice, patients are customarily offered neoadjuvant chemoradiation for borderline disease, and then imaged again to determine whether downstaging to resectability is possible (Figure 5). Changes in the appearance of irradiated tissue often mimic tumor infiltration, complicating assessment of treatment response, leading to frustration for the radiologist, and uncertainty for the entire management team as to the appropriate next step. Whether initially resectable, or downstaged through non-operative treatment, a detailed map of the local blood vessel anatomy must be created, especially any variants, such as a replaced right hepatic artery, which will usually course through the surgical field of a Whipple resection (Figure 6). These vascular issues become even more important when a laparoscopic surgical approach, with its limited field-of-view, is intended.
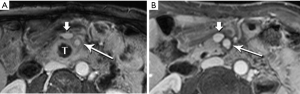
Moving beyond local staging, the imaging hunt for metastatic disease will focus on the liver, by far the most likely site, and screen for other sites of disease, such as the peritoneum (Figure 7). Discovery of liver metastases is critical to optimal management, to avoid treatment delay and the morbidity of surgery undertaken with the false assumption that the tumor is locally confined. It has been shown that occult liver metastases are often present at the time of pancreatectomy (3).
Multiphase computed tomography (CT) for detection, staging, and post-treatment surveillance of PDA has received much attention in the literature over the past several decades, establishing itself as the gold standard imaging modality (4,5). Given the wide availability of high-quality CT systems and imaging protocols that are similar from institution to institution, current NCCN guidelines give CT first-line status for imaging work-up, with the caveat that high quality technique is required (6). Since PDA is generally a hypoenhancing tumor, at least two phases obtained following rapid bolus contrast injection (3-4 mL/sec) are required. The first, or pancreatic phase, is obtained at optimal pancreatic parenchymal enhancement, and the second, or portal phase, at optimal liver enhancement (7). In both phases, then, the primary tumor and any possible metastases will appear hypoattenuating to the background tissue. MRI is considered acceptable, but not preferable, due to expense, as well as the variable quality and expertise available in the radiology community. MRI protocols are also not currently standardized, and are not likely to be so in the near future, at least outside of academic centers and other high-level imaging practices. A role for positron emission tomography (PET) has not been established for a variety of reasons. For example, Wang et al. found that maximum standardized uptake value (SUVmax) of benign and malignant pancreatic lesions overlapped significantly in benign and malignant cases (8). Moreover, NCCN guidelines state that PET/CT is not a substitute for high-quality, contrast-enhanced CT. Addressing endoscopic ultrasound (EUS), the NCCN does not accord the procedure the status of a routine staging tool, but does recognize that EUS-directed biopsy is preferable to biopsy under CT (2,9).
In our institution, discussion and decisions regarding PDA management for individual patients occur in weekly multidisciplinary conferences. Accumulated experience in the group has led to fairly routine incorporation of EUS, not only for biopsy, but for tasks such as sampling of mucinous tumors, and clarification when CT or MRI is inconclusive or technically suboptimal. In addition, MRI has over the years become the primary imaging modality for diagnosis and staging of PDA for our patients.
While CT has always benefited from excellent spatial resolution, that is, the ability to depict fine structures and their relationships, it is eclipsed by MRI in the arena of contrast resolution, that is, the wide and manipulable gray scale that brings out differences between tissues, differences that may be subtle or indiscernible to CT. For example, it is contrast resolution that gives MRI its greater sensitivity to small liver metastases, which in turn may justify its additional cost. A recent study concluded that MRI outperforms multidetector CT for pancreatic metastases to the liver (10). Similarly, a meta-analysis found MRI superior to CT, FDG-PET, and PET-CT for hepatic metastases from gastrointestinal primaries (11). The improved contrast resolution of MRI is also responsible for the ease with which it can depict very small ducts and cystic lesions, which are markedly bright on heavily T2-weighted, fluid-sensitive techniques, popularly known as MR cholangiopancreatography (MRCP).
Required for PDA imaging with MRI is a multiphase contrast-enhanced protocol, using the same approach established for CT. Unique to MRI, diffusion-weighted imaging (DWI) has recently become sufficiently robust to be effective in the abdomen, and brings an exquisite sensitivity for most hepatic metastases (Figure 8). Fast, single-shot T2-weighted imaging, including MRCP, provides the final piece of vital information—the status of fluid-filled structures, whether pancreatic duct, bile duct, cystic lesions, or necrosis. An MRCP acquisition will also nicely demonstrate the classic double duct sign of a pancreatic head mass, where the desmoplastic, obstructing mass causes dilation of the ducts upstream (Figure 9). These three techniques form the bedrock of MRI imaging for PDA.
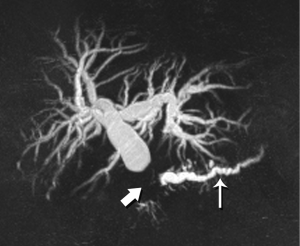
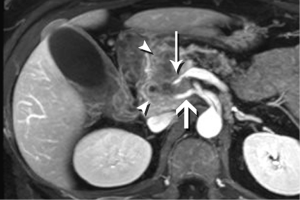
Although the literature on staging emphasizes the relationship of tumor with the regional arteries, veins are often equally problematic It is true that even complete encasement of the PV is not considered an absolute contraindication to resection, unless technical issues impede the surgeon. However, in the setting of SMV invasion, we often discover that the tumor also involves critical jejunal tributaries or the inferior mesenteric vein, precluding successful surgical reconstruction. The anatomy of these vessels is also quite variable, so clear visualization is critical.
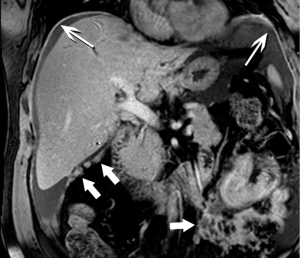
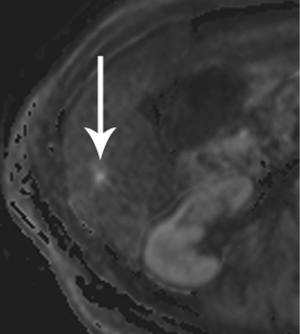
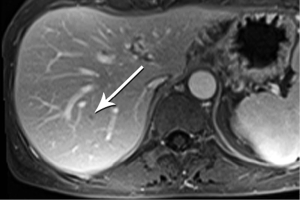
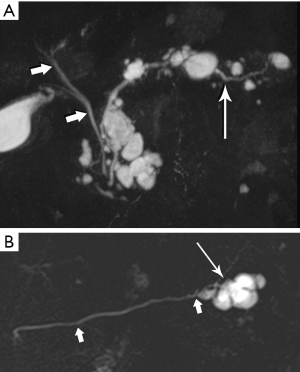
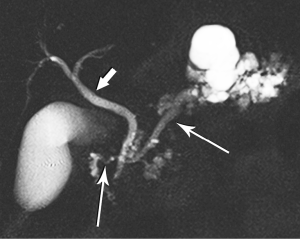
High-quality venous imaging has traditionally been elusive for conventional contrast-enhanced imaging, whether CT or MRI. Both iodinated contrast (for CT) and gadolinium-based contrast (MRI) are extravascular agents, that is, they quickly exit the blood pool at the tissue capillary bed. This is how tissue enhancement is accomplished, but it also means that beyond the arterial phase tissue enhancement tends to obscure vessels, especially small ones, and especially in areas where there is little separation of structures, like the pancreas and neighboring structures. To address this challenge, we have devised a novel approach to our PDA imaging that employs two different contrast agents. We first administer gadofosveset trisodium (AblavarR, Lantheus Medical Imaging, Billerica, MA, USA), a commercially available gadolinium-based agent that circulates in the blood pool for a prolonged period due to reversible binding with serum proteins. We then obtain magnetic resonance arteriogram and venogram (MRA/MRV) sequences in the coronal and axial planes using a hybrid technique that retains more information about tissue than conventional MRA, which is traditionally designed to exclude non-vascular structures as much as possible. When our conventional contrast agent is then injected, and subsequent imaging performed, the vessels, even smaller veins like jejunal tributaries and pancreaticoduodenal veins, retain their conspicuity in the context of the primary tumor and any extrapancreatic infiltration (Figure 10). An unexpected bonus has been visualization of liver metastases on the order of 3-4 mm in diameter, likely because the hybrid MRA slice thickness is between 1-2 mm (Figure 11). Finally, all of our PDA patients are scheduled on a 3 Tesla scanner to optimize spatial resolution.
While improvements in imaging technology provide increasingly reliable guidance regarding the presence and behavior of PDA, detection and staging remain extremely challenging, mirroring the intractability of this tumor when it comes to treatment. Incremental improvements should continue as technologies like PET-MR become more mature and novel molecular imaging agents become available. For the time being, however, it is clear that imaging and clinical management of PDA should occur in high volume centers with subspecialty expertise and state-of-the-art technology (7,12).
Acinar cell carcinoma
Although the second most common malignancy of the exocrine pancreas, acinar cell carcinoma is still quite rare, accounting for only 1-2% of these malignancies. It differs in both imaging appearance and prognosis from ductal adenocarcinoma, with improved outcomes (13,14). Acinar cell carcinoma is also famous for its ability to produce dramatically high levels of circulating lipase, resulting in an impressive clinical presentation involving painful subcutaneous nodules due to fat necrosis, polyarthropathy, and eosinophilia. However, this presentation occurs only in approximately 15% of cases, with non-specific weight loss and abdominal pain more often the presenting symptoms (14).
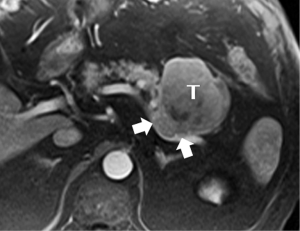
Relatively soft and well-circumscribed, at least in part because it does not elaborate the desmoplastic extracellular matrix that is so prominent a feature of PDA, the acinar cell tumor tends not to have an infiltrative appearance, but rather to appear as a solid mass, with varying amounts of intralesional necrosis (15). Its typically fairly large size at presentation may be due to delayed diagnosis made possible by this lack of infiltrative behavior. Enhancement also tends to be greater than the typical low-level enhancement of a ductal tumor, and duct dilation less common (Figure 12).
Pseudopapillary tumor of the pancreas (PPT)
The uncommon, but not rare, PPT occupies a unique niche in the spectrum of pancreatic tumor due to its very specific demographic: young women. In a recent study of 97 patients with PPT, only 4 were male, and mean age in most reports is early 30’s (16). A low-grade malignancy, PPT rarely metastasizes, and local invasion is not a common feature. The large majority benefit from with surgical management, which is universally recommended, with resolution of symptoms and no recurrence (17-19). Unlike PDA, these are well-defined tumors, although they lack a real capsule, and they are more likely to occur in the tail and body than in the head. Heterogeneity is a very common feature, largely because of cystic elements, but with hemorrhage also recognized in 25-30% (20,21). Cystic elements are appreciated through their high signal intensity on T2-W images, and hemorrhage by the similarly high signal on T1-W images (Figure 13). Enhancement is variable, but tends to be progressive. Smaller tumors tend to behave somewhat differently, favoring a more homogeneous appearance on unenhanced imaging, but with early heterogeneous and progressive enhancement (22). Little has been reported about PPT’s appearance on DWI, but our institutional experience is that these lesions demonstrate significantly higher signal intensity on high b-value DWI than do PDA or mucinous pancreatic tumors. As these lesions enlarge, recognition of their origin in the pancreas becomes critical, especially those in the body and tail, so as not to mistake them for gastric GIST, adrenal lesions, or splenic masses.
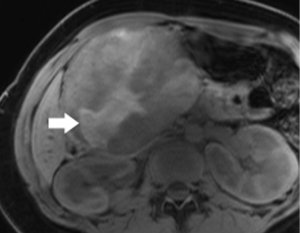
Pancreatic neuroendocrine tumor (PNET)
Although a functional PNET can present clinically with dramatic symptoms due to hypersecretion of one of several digestive hormones, the majority of these tumors are non-functional. Similarly, although often multiple when associated with genetic syndromes, especially multiple endocrine neoplasia type 1 (MEN-1), most PNET are solitary and sporadic (23). Reports regarding biologic behavior vary. The largest cohort has been compiled from the surveillance, epidemiology, and end results (SEER) registry. Among this group of nearly 1,500 PNET, the majority were malignant, with 60% metastatic at diagnosis, and 21% locally invasive, with prognosis somewhat improved for the functional variants (24). On the other hand, a single institution’s experience with 168 surgically resected PNETs found that more than three-quarters were benign, and half were found incidentally (25). Since they are symptomatic, functional tumors tend to be smaller when discovered, especially insulinomas.
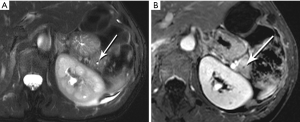
The classic imaging appearance of functional PNET has been described as solid, well-circumscribed and hypervascular, which may be explained by the dense network of vessels encompassing the islet cells of the pancreas, required by the physiologic demand for a rapid supply of these hormones during the course of digestion. Imaging protocols have been devised to take advantage of these characteristics. A contrast-enhanced CT or MRI acquisition obtained in the arterial or pancreatic phase has long been considered critical for lesion detection, especially for the smaller, more solid tumors (Figure 14). For example, in a review of 30 resected insulinomas, only 63% were initially recognized on contrast-enhanced CT. However, in the subgroup of tumors that had undergone focused CT that had incorporated a pancreatic phase, all were recognized (26). Our MR experience with PNETs has been that a minority of these lesions can be frustratingly subtle with even the best designed imaging protocols, especially in the setting of MEN-1, due both to their usually small size and to their multiplicity (Figures 15,16). Also, although EUS has proven quite useful for detection and biopsy, we have found that even meticulous EUS often misses one or more lesions.
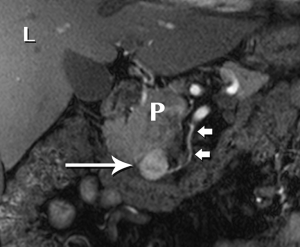
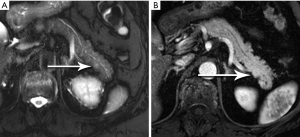

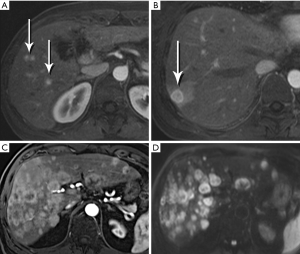
Nonfunctional tumors have a more varied appearance, with hypoenhancing elements, necrosis, and invasive behavior developing as the lesions enlarge (Figures 17,18). Generally, PNETs are only mildly hyperintense on T2W images, with the exception of cystic components, which will be strongly hyperintense (27). Despite the fact that these tumors are generally thought of as solid masses, as many as 18% of PNETs less than 3 cm are predominately cystic (28), and when small lesions become cystic, they can easily be mistaken for side-branch intraductal papillary mucinous neoplasms (IPMN) or pseudocyst (Figure 19). The liver is by far the most likely target of metastases from PNETs, where the lesions are hyperenhancing and usually multiple (Figure 20). DWI sequences are exquisitely sensitive to the presence of liver metastases, out-performing T2-W and dynamic contrast-enhanced techniques alike, and should routinely be included in MR scanning protocols for PNETs (29).
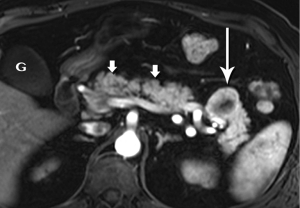
Intraductal papillary mucinous neoplasm (IPMN)
IPMN has attracted a remarkable, and increasing, amount of attention over the past 15-20 years. To a great degree, the still-escalating utilization of ever-more sophisticated imaging technologies has been responsible for the progressive increase in detection of these lesions. The sub-millimeter spatial resolution now available on multidetector CT (MDCT) systems and the excellent sensitivity of high-level MR systems for sub-centimeter cysts are the most important culprits. For example, in 2008, Laffan et al. reported a 2.6% prevalence of incidental pancreatic cysts found on MDCT (30). In a more recent study we have found an overall 43% incidental cyst prevalence in 500 patients undergoing abdominal MRI at our institution over a 10-year period, with the rate increasing during that time (31).
The defining feature of IPMN is intraductal accumulation of mucin produced by plaque-like tumors arrayed along the epithelial surface of the main pancreatic duct, its side branches, or both. As mucin accumulates, the affected duct dilates, sometimes to a remarkable degree, resulting in symptoms ranging from abdominal pain to pancreatitis (Figures 21,22,23). In early years, this entity was an endoscopic diagnosis made by recognition of mucin protruding into the duodenum through a bulging papilla (32), usually representing an advanced lesion.
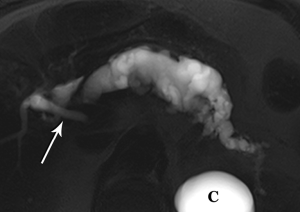
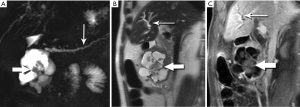
In conjunction with increased and earlier detection, recognition of the malignant potential of IPMN lesions has led to confusion and controversy, with vacillating approaches to management. Intensive research efforts by the surgical, gastroenterology, and radiology communities are at last bringing some clarity to the conversation. One very large step has been taken with the recognition that main duct (md) and branch duct (bd) IPMN appear to be distinct lesions, given their markedly different rates of progression to frank malignancy and very different patient outcomes (33-35). In the past few years, in recognition of their very indolent growth pattern, simple imaging surveillance has become the primary management choice for small (<3 cm) suspected bd-IPMNs that lack suspicious features, enhancing mural nodules, or rapid growth in size (36-38).
Of greater concern are main duct or mixed main duct/side branch IPMN. The risk of malignant transformation appears to be an order of magnitude higher than for isolated bd-IPMN, and thus surgical resection or close surveillance are more often the appropriate management options. Consensus guidelines published in 2012 consider a main duct diameter of 10 mm or greater to be of high risk for malignancy, with 5-10 mm classified as worrisome (37). Enhancing nodules and obstructive jaundice are the other two high-risk stigmata. Even in the presence of stigmata of chronic pancreatitis, a main duct with a diameter of 5 mm or greater is worrisome (39).
High-resolution MRCP imaging is not only exquisitely sensitive to the presence of fluid, but it can also demonstrate the internal features of cystic lesions, such as locularity, septations, alterations in cyst fluid characteristics, and communication with the main duct, and is thus ideal for purposes of follow up (Figure 24). When combined with contrast-enhancing imaging, MR can distinguish between solid neoplastic mural nodules and clumps of non-enhancing inspissated mucin, and can detect enhancement of duct wall and septations. In a study of 51 patients with surgically resected md-IPMN who had undergone MRI/MRCP (40), Manfredi et al. found that enhancing mural nodules and diffuse duct involvement were most predictive of malignancy (Figures 25,26).
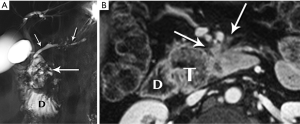
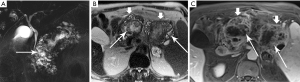
Mucinous cystic neoplasm (MCN)
Another unique pancreatic tumor, the MCN is also a gender-specific entity: in a classic study of 130 MCN, all patients were women (41). This demographic distribution ceased to be surprising when ovarian stroma was recognized as a characteristic and requisite feature for diagnosis of this tumor. Although slow-growing and rather indolent, all MCN are considered to have malignant potential. With a mean age of 45 years, patients tend to be older than those with PPT. Their tumors are typically large, with a mean diameter of 10 cm, and nearly all are in the pancreatic body/tail (Figure 27). Primarily cystic, without internal hemorrhage, they contain mucin rather than serous fluid.
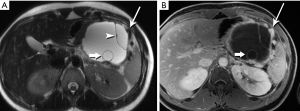
Recognition of the small mural nodules, which is where the dysplastic/neoplastic elements reside, is challenging for CT, but facilitated in MRI by the marked contrast between fluid and tissue (Figure 28). Subtraction of the pre-contrast from the post-contrast images will confirm that high signal foci represent enhancing tissue rather than hemorrhage or inspissated mucin. MRI readily characterizes the oligocystic nature of this tumor, the well-circumscribed wall, the very common small mural nodules, and the lack of hemorrhage or communication with the duct (42,43). Unless these features are clearly demonstrated on imaging, an MCN lesion is easily mistaken for a pseudocyst.
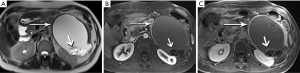
Pitfalls
Primary pancreatic malignancies can be mimicked by a handful of other entities, including metastases from elsewhere, inflammatory processes, ampullary and bile duct tumors, and anatomic variants like intrapancreatic splenic tissue.
Metastases to the pancreas are quite uncommon, with a smattering of reports involving breast, colorectal, lung, and cutaneous melanoma primaries. By far the most common are of renal cell carcinoma (RCC) origin (44). Interestingly, these tend to be fairly indolent, perhaps due to a lower biologic aggressiveness of the primary, with generally excellent survival data, even when diagnosis is delayed (45-47). Metastases from RCC are recognized for a hypervascularity that results in striking early hyperenhancement similar to the appearance of a primary clear cell RCC, but also often not distinguishable from a PNET (Figure 29). Because of this behavior, inclusion of an arterial phase sequence following contrast injection is rewarded by greater tumor conspicuity against a background of more gradually enhancing pancreatic parenchyma (48). In our experience, multiple metastases occur more commonly than solitary lesions (Figure 30).
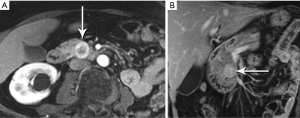
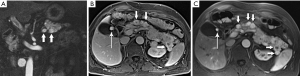
IgG4-related sclerosing disease is an umbrella term that refers to a family of fibrosing disorders characterized by tissue infiltration by IgG4 rich plasma cells. Potentially affected organs include kidneys, biliary tree, salivary glands, small bowel mesentery, and pancreas, alone or in combination. Because the disease often affects the pancreas focally, with swelling, decreased enhancement, and stricturing of involved ducts, IgG4-related pancreatitis can mimic pancreatic adenocarcinoma (Figure 31). Also diagnostically problematic is the absence of gland edema and peri-pancreatic fluid so typical of inflammatory pancreatitis. Although a good deal of attention has been paid in recent years to this entity, IgG4-related pancreatitis is still under-recognized, resulting in major surgeries and other unnecessary treatments for a disease that typically responds quite rapidly to steroids. Observations like focally increased bulk without infiltration beyond the gland; uniform low level enhancement; a narrowed and irregular, but not obstructed, duct; delayed capsule-like enhancement; and marked diffusion restriction should stimulate consideration of focal IgG4-related pancreatitis as an alternative to malignancy (49-52).
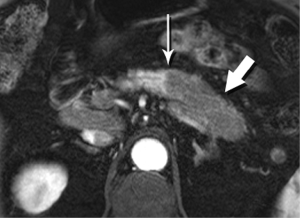
Splenules, or accessory spleens, are very common incidental findings on cross-sectional imaging. Since they are most often found in the vicinity of the splenic hilum, they are also in close proximity to the pancreatic tail, sometimes actually within the substance of the tail. An intrapancreatic splenule can mimic a hypervascular pancreatic mass, such as a neuroendocrine tumor. Fortunately, a splenule will match the T2-W appearance, the diffusion behavior, and the very characteristic enhancement pattern of the spleen itself (Figure 32).
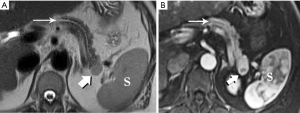
Ampullary carcinoma arises from the glandular epithelium of the ampulla of Vater and, like duodenal carcinoma, carries a more favorable prognosis than PDA (53). However, it is easily mistaken for carcinoma of the head of the pancreas since obstruction at the level of the ampulla will also produce the double duct sign for which PDA is so well known. It also tends to be similarly hypoenhancing, ill-defined, and small at presentation (Figure 33). Similar confusion can be created by cholangiocarcinoma affecting the distal, intrapancreatic portion of the bile duct.

Conclusions
Despite incremental improvements in diagnostic efficacy of imaging for cancer of the pancreas, detection, characterization, and staging of these lesions remain challenging. These challenges mirror the difficulties currently faced by the oncologists, gastroenterologists, and surgeons who are charged with managing the patients afflicted by pancreatic tumors. CT, MRI, and FDG-PET have roles that vary by institution, rightly so because levels of expertise vary. In summary, however, the patient will be best served by diagnostic imaging performed in high volume centers with state-of-the art technology (12). Perhaps even more important is the availability of interested sub-specialist radiologists who are educated to the oncologic and surgical issues critical to effective treatment of this stubborn, too-often lethal disease.
For the time being, however, it is clear that imaging and clinical management of PDA should occur in high volume centers with subspecialty expertise and state-of-the-art technology (7,12).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (R. Charles Nichols Jr, Debashish Bose and George P. Kim) for the series “Pancreatic Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.11.05). The series “Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Croome KP, Jayaraman S, Schlachta CM. Preoperative staging of cancer of the pancreatic head: is there room for improvement? Can J Surg 2010;53:171-4. [PubMed]
- Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2012;10:703-13. [PubMed]
- Amikura K, Kobari M, Matsuno S. The time of occurrence of liver metastasis in carcinoma of the pancreas. Int J Pancreatol 1995;17:139-46. [PubMed]
- Karmazanovsky G, Fedorov V, Kubyshkin V, et al. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging 2005;30:488-500. [PubMed]
- Quiney B, Harris A, McLaughlin P, et al. Dual-energy CT increases reader confidence in the detection and diagnosis of hypoattenuating pancreatic lesions. Abdom Imaging 2015;40:859-64. [PubMed]
- Tempero MA, Malafa MP, Asbun H, et al. National Comprehensive Cancer Networks. Pancreatic Adenocarcinoma, version 2.2015: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2015;18.
- Fletcher JG, Wiersema MJ, Farrell MA, et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology 2003;229:81-90. [PubMed]
- Wang XY, Yang F, Jin C, et al. Utility of PET/CT in diagnosis, staging, assessment of resectability and metabolic response of pancreatic cancer. World J Gastroenterol 2014;20:15580-9. [PubMed]
- Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 2012;75:319-31. [PubMed]
- Motosugi U, Ichikawa T, Morisaka H, et al. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology 2011;260:446-53. [PubMed]
- Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 2010;257:674-84. [PubMed]
- Walters DM, Lapar DJ, de Lange EE, et al. Pancreas-protocol imaging at a high-volume center leads to improved preoperative staging of pancreatic ductal adenocarcinoma. Ann Surg Oncol 2011;18:2764-71. [PubMed]
- Matos JM, Schmidt CM, Turrini O, et al. Pancreatic acinar cell carcinoma: a multi-institutional study. J Gastrointest Surg 2009;13:1495-502. [PubMed]
- Klimstra DS, Heffess CS, Oertel JE, et al. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol 1992;16:815-37. [PubMed]
- Tatli S, Mortele KJ, Levy AD, et al. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am J Roentgenol 2005;184:511-9. [PubMed]
- Yu P, Cheng X, Du Y, et al. Solid Pseudopapillary Neoplasms of the Pancreas: a 19-Year Multicenter Experience in China. J Gastrointest Surg 2015;19:1433-40. [PubMed]
- Yagcı A, Yakan S, Coskun A, et al. Diagnosis and treatment of solid pseudopapillary tumor of the pancreas: experience of one single institution from Turkey. World J Surg Oncol 2013;11:308. [PubMed]
- Reddy S, Cameron JL, Scudiere J, et al. Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. J Am Coll Surg 2009;208:950-7; discussion 957-9. [PubMed]
- Li G, Baek NH, Yoo K, et al. Surgical outcomes for solid pseudopapillary neoplasm of the pancreas. Hepatogastroenterology 2014;61:1780-4. [PubMed]
- Ventriglia A, Manfredi R, Mehrabi S, et al. MRI features of solid pseudopapillary neoplasm of the pancreas. Abdom Imaging 2014;39:1213-20. [PubMed]
- Yao X, Ji Y, Zeng M, et al. Solid pseudopapillary tumor of the pancreas: cross-sectional imaging and pathologic correlation. Pancreas 2010;39:486-91. [PubMed]
- Yu MH, Lee JY, Kim MA, et al. MR imaging features of small solid pseudopapillary tumors: retrospective differentiation from other small solid pancreatic tumors. AJR Am J Roentgenol 2010;195:1324-32. [PubMed]
- Hoff A, Cote G, Gagel R. Management of neuroendocrine cancers of the gastrointestinal tract: islet cell carcinoma of the pancreas and other neuroendocrine carcinomas. In: Abbruzzese J, Evans D, Willett C, et al., editor(s). Gastrointestinal oncology. New York: Oxford University Press, 2004:780-800.
- Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727-33. [PubMed]
- Vagefi PA, Razo O, Deshpande V, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg 2007;142:347-54. [PubMed]
- Fidler JL, Fletcher JG, Reading CC, et al. Preoperative detection of pancreatic insulinomas on multiphasic helical CT. AJR Am J Roentgenol 2003;181:775-80. [PubMed]
- Buetow PC, Parrino TV, Buck JL, et al. Islet cell tumors of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR Am J Roentgenol 1995;165:1175-9. [PubMed]
- Kawamoto S, Johnson PT, Shi C, et al. Pancreatic neuroendocrine tumor with cystlike changes: evaluation with MDCT. AJR Am J Roentgenol 2013;200:W283-90 [PubMed]
- d'Assignies G, Fina P, Bruno O, et al. High sensitivity of diffusion-weighted MR imaging for the detection of liver metastases from neuroendocrine tumors: comparison with T2-weighted and dynamic gadolinium-enhanced MR imaging. Radiology 2013;268:390-9. [PubMed]
- Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 2008;191:802-7. [PubMed]
- Moris M, Bridges M, Pooley R, et al. Advances in high resolution cross sectional imaging and the rising prevalence of pancreatic cysts over the past decade. Available online: https://cdn.ueg.eu/ueg-week-2015/posters-and-videos/P1341.pdf
- Procacci C, Graziani R, Bicego E, et al. Intraductal mucin-producing tumors of the pancreas: imaging findings. Radiology 1996;198:249-57. [PubMed]
- Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol 2000;24:1372-7. [PubMed]
- Kim TH, Song TJ, Hwang JH, et al. Predictors of malignancy in pure branch duct type intraductal papillary mucinous neoplasm of the pancreas: A nationwide multicenter study. Pancreatology 2015;15:405-10. [PubMed]
- Pezzilli R, Calculli L. Branch-type intraductal papillary mucinous neoplasm of the pancreas: clinically and patient-reported outcomes. Pancreas 2015;44:221-6. [PubMed]
- Lee CJ, Scheiman J, Anderson MA, et al. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg 2008;12:234-42. [PubMed]
- Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183-97. [PubMed]
- Shin SS, Armao DM, Shah M, et al. Management of branch-duct intraductal papillary mucinous neoplasms of the pancreas: observation with MR imaging. Magn Reson Imaging 2010;28:1440-6. [PubMed]
- Talamini G, Zamboni G, Salvia R, et al. Intraductal papillary mucinous neoplasms and chronic pancreatitis. Pancreatology 2006;6:626-34. [PubMed]
- Manfredi R, Graziani R, Motton M, et al. Main pancreatic duct intraductal papillary mucinous neoplasms: accuracy of MR imaging in differentiation between benign and malignant tumors compared with histopathologic analysis. Radiology 2009;253:106-15. [PubMed]
- Thompson LD, Becker RC, Przygodzki RM, et al. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: a clinicopathologic study of 130 cases. Am J Surg Pathol 1999;23:1-16. [PubMed]
- Plichta JK, Brosius JA, Pappas SG, et al. The changing spectrum of surgically treated cystic neoplasms of the pancreas. HPB Surg 2015;2015:791704.
- Manfredi R, Ventriglia A, Mantovani W, et al. Mucinous cystic neoplasms and serous cystadenomas arising in the body-tail of the pancreas: MR imaging characterization. Eur Radiol 2015;25:940-9. [PubMed]
- Moussa A, Mitry E, Hammel P, et al. Pancreatic metastases: a multicentric study of 22 patients. Gastroenterol Clin Biol 2004;28:872-6. [PubMed]
- Sohn TA, Yeo CJ, Cameron JL, et al. Renal cell carcinoma metastatic to the pancreas: results of surgical management. J Gastrointest Surg 2001;5:346-51. [PubMed]
- Faure JP, Tuech JJ, Richer JP, et al. Pancreatic metastasis of renal cell carcinoma: presentation, treatment and survival. J Urol 2001;165:20-2. [PubMed]
- Kalra S, Atkinson BJ, Matrana MR, et al. Prognosis of patients with metastatic renal cell carcinoma and pancreatic metastases. BJU Int 2015; [Epub ahead of print]. [PubMed]
- Jain Y, Liew S, Taylor MB, et al. Is dual-phase abdominal CT necessary for the optimal detection of metastases from renal cell carcinoma? Clin Radiol 2011;66:1055-9. [PubMed]
- Frulloni L, Scattolini C, Falconi M, et al. Autoimmune pancreatitis: differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol 2009;104:2288-94. [PubMed]
- Takuma K, Kamisawa T, Gopalakrishna R, et al. Strategy to differentiate autoimmune pancreatitis from pancreas cancer. World J Gastroenterol 2012;18:1015-20. [PubMed]
- Sahani DV, Kalva SP, Farrell J, et al. Autoimmune pancreatitis: imaging features. Radiology 2004;233:345-52. [PubMed]
- Muhi A, Ichikawa T, Motosugi U, et al. Mass-forming autoimmune pancreatitis and pancreatic carcinoma: differential diagnosis on the basis of computed tomography and magnetic resonance cholangiopancreatography, and diffusion-weighted imaging findings. J Magn Reson Imaging 2012;35:827-36. [PubMed]
- Sarmiento JM, Nagomey DM, Sarr MG, et al. Periampullary cancers: are there differences? Surg Clin North Am 2001;81:543-55. [PubMed]


