
Effects of brucine on mitochondrial apoptosis and expression of HSP70 in prostate cancer cells
Introduction
Prostate cancer is a common malignancy of the male reproductive system, and its incidence is highest in some European countries (1). Prostate cancer has a rapid progression and is prone to metastasis. Most patients are already in the middle and advanced stages when it is detected, significantly affecting their physical and mental health (2). Currently, surgery is the primary clinical treatment for prostate cancer, and postoperative radiotherapy and chemotherapy are also essential; however, the side effects of radiotherapy and chemotherapy drugs greatly reduce patients’ quality of life. Therefore, the search for a new therapeutic target and drug is essential to reduce the development of prostate cancer and improve the quality of life for prostate cancer patients. Some researchers used modern molecular biotechnology to determine the main anti-tumor target of Brucine, obtained the crystal structure of the complex between the main active components and the target protein, and completed the anti-tumor activity test in mice. Through in vivo and in vitro activity screening, it was found that the alkaloids in Tibetan Baltic flower have good antitumor activity in vivo and in vitro. This not only provides experimental reference for the further development of brucine anti-tumor drugs, but also provides a new molecular template for the research and development of anti-tumor drugs, and lays a theoretical foundation for the exploration and promotion of the medicinal value of brucine anti-tumor drugs in the later stage.
Strychnos nux-vomica is the dried seed of the Strychnos plant and is effective in relieving pain, dispersing muscular knots, and reducing swelling. Brucine is a major active ingredient in strychnos nux-vomica and is considered by modern medicine to have analgesic, anti-inflammatory, central nervous system stimulating, immune boosting, and antitumor effects (3). In recent years, brucine has gained widespread attention for its antitumor properties. Previous studies have indicated that brucine can regulate apoptosis in skin cancer and breast cancer, thus acting as an antitumor agent (4,5). Heat shock protein 70 (HSP70) is a highly conserved protein shown to be involved in tumor cell apoptosis via the mitochondrial apoptosis pathway. Silencing HSP70 expression has been shown to induce apoptosis in prostate cancer PC-3 cells. Whether brucine can regulate apoptosis in prostate cancer cells through the expression of HSP70 is not yet known. Therefore, this study preliminarily investigated the effects of brucine on mitochondrial apoptosis and HSP70 expression in prostate cancer cells, and the possible mechanisms were analyzed in the hope of providing an experimental basis for the clinical application of brucine. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-209/rc).
Methods
Cell lines
We used the human prostate cancer PC-3 cell line (bio-72942) purchased from ATCC, USA.
Main reagents and equipment
The main reagents used in the study were brucine (Chengdu Manster Biotechnology Co. Ltd., China), RPMI-1640 medium (Hyclone, USA), fetal bovine serum (FBS) (Zhejiang Tianhang Biotechnology Co. Ltd., China), an methodology of tetrazolium (MTT) cell viability assay kit (Nanjing Jiancheng Biotechnology Co., Ltd., China), anti-HSP70 antibody (ab30342; Abcam, USA), anti-apoptotic protease activating factor 1 (Apaf-1) antibody (ab31392; Abcam, USA), anti-cysteine protease-3 (caspase-3) antibody (ab31383; Abcam, USA), and β-actin rabbit anti-polyclonal antibody (ab33563; Abcam, USA).
The equipment used included a CO2 cell incubator (Thermo Scientific, Germany, model BB-15), a multifunctional biological microscope (Olympus Optical Ltd., Japan, model OLYMPUS BX-51), a tabletop high-speed frozen centrifuge (Eppendorf, Germany), and an enzyme labeler (Thermo Scientific, Thermo MULTISKANFC, Germany).
Cell culture
The purchased human prostate cancer PC-3 cells were thawed and revived, then inoculated in the 1640 medium containing 10% FBS and 100 U/mL penicillin for culture. The CO2 cell culture incubator conditions were as follows: temperature 37 °C, CO2 concentration 5%, and humidity 95%. When the cells achieved a density of approximately 80–90%, they were treated with 0.25% trypsin 2 mL, at a ratio of 1:3 for passaged culture.
MTT assay for cell viability
PC-3 cells at the logarithmic growth stage were inoculated into 96-well plates at a density of 1×104 cells/mL. After 24 h of incubation in a CO2 incubator at 37 °C, with a CO2 concentration of 5%, and humidity of 95%, 100 μL of differing brucine solution concentrations (0, 25, 50, and 100 μmol/L) was added to the 96-well plates with six replicate wells for each concentration. The concentrations were designated as the control group (0 μmol/L), low dose brucine group (25 μmol/L), medium dose brucine group (50 μmol/L), and high dose brucine group (100 μmol/L). After 24 and 48 h of incubation, MTT (10 μL at a concentration of 5 mg/mL) was added, and the cells were incubated for a further 4 hours. The supernatant was then discarded, and DMSO (150 μL) was added. The cell viability was calculated by measuring the OD (optical density) of each well at 490 nm by enzyme-linked immunoassay. Cell viability (%) was established by the following formula: OD of the experimental group/OD of the control group × 100%.
Hoechst 33258 staining
PC-3 cells in the logarithmic growth phase (1×104 cells/mL) were inoculated on treated coverslips and incubated in 6-well plates for 24 h. Brucine was added at concentrations of 25, 50, and 100 μmol/L in the experimental group, and an equal volume of solvent DMSO was added to the control group. After 48 h, they were fixed with 4% formaldehyde for 10 min, washed twice with PBS, and 0.5 mL Hoechst 33258 staining solution was added under dry and dark conditions. The cells were incubated for 30 min in the dark, washed twice with PBS, and one drop of fluorescence quenching agent was added before sealing the coverslip. The cell apoptosis was observed under a fluorescence microscope.
Flow cytometry assays
Human prostate cancer PC-3 cells in the logarithmic growth phase were inoculated into 6-well plates, treated with different drugs after complete adherent growth, and incubated for 48 h. The cells were then digested with trypsin, centrifuged at 1,000 rpm for 5 min at 4 °C, and washed twice with PBS. They were then resuspended in 300 μL of binding buffer, mixed with 10 μL Annexin V-FITC and incubated in the dark for 15 min. Subsequently, the cells were supplemented with 5 μL propidium iodide (PI), incubated at 4 °C for 5 min protected from light, then filtered using a 200-mesh cell sieve. The apoptotic rate of each group of cells was then measured by flow cytometry.
Western blot detection of HSP70, Apaf-1, and caspase-3 protein expression levels in PC-3 cells
The PC-3 cells from each group were lysed on ice with protein lysis solution and protease inhibitor and centrifuged at 12,000 r/min for 20 min at 4 °C. The supernatant was removed, and the protein content was quantified using a BCA kit. The protein samples (40 μg) were then separated on a 10% SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membranes, blocked at room temperature for 2 h with 5% skimmed milk powder and incubated overnight at 4 °C with the primary antibodies HSP70, Apaf-1, caspase-3, and β-actin. The membranes were subsequently washed with TBS-T and incubated with goat anti-rabbit IgG secondary antibody. Protein bands were detected by enhanced chemiluminescence reagents, and the protein expression ratios were analyzed.
Statistical analysis
SPSS 22.0 software was used for all statistical analyses. The measurement data were described by the mean and standard deviation (), and one-way analysis of variance (ANOVA) and t-tests were used for the between-group comparisons. A P value <0.05 was considered to be statistically significant.
Results
Effect of brucine on the activity of PC-3 cells
As shown in Table 1, at 24 and 48 h, PC-3 cell activity was significantly lower in the low, medium, and high dose brucine groups than in the control group (P<0.01) and decreased with an increased dose.
Table 1
| Groups | 24 h | 48 h |
|---|---|---|
| Control group | 98.24±2.23 | 98.41±1.73 |
| Brucine low dose group | 91.13±4.81** | 90.13±6.35**# |
| Brucine medium dose group | 88.23±4.39** | 81.27±5.72**## |
| Brucine high dose group | 84.23±8.17** | 71.23±4.34**## |
**P<0.01 compared to control. #P<0.05 and ##P<0.05 for each group compared to control at 24 h.
Effect of brucine on nuclear morphology and apoptosis of PC-3 cells
Hoechst is a specific class of DNA dye that, under normal conditions, can pass through the cell membrane in small amounts. When bound to DNA, the Hoechst stain gives the nucleus a diffuse and uniform low blue fluorescence. When apoptosis occurs, the permeability of the cell membrane increases, allowing more dye to pass through, and when bound to DNA, the Hoescht stain gives the nucleus a deeper blue fluorescence. In this study, the nuclei of the control group fluoresced uniformly with intact nuclei. The nuclei of the brucine groups appeared crinkled, and dense granular blocks of strong blue fluorescence were visible (Figure 1).
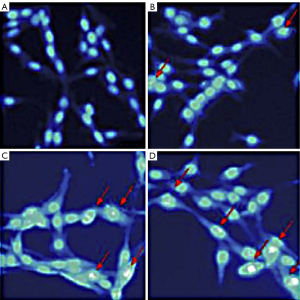
Effect of brucine on the apoptosis of PC-3 cells
The apoptosis rate of PC-3 cells in the low, medium, and high dose brucine groups was significantly higher than that in the control group, and the difference was significant (P<0.01). The apoptosis rate of the PC-3 cells increased significantly with an increased dose, showing a dose-dependent effect (Table 2 and Figure 2).
Table 2
| Groups | Apoptosis rate |
|---|---|
| Control group | 7.33±1.27 |
| Brucine low dose group | 15.33±2.49** |
| Brucine medium dose group | 23.25±2.21** |
| Brucine high dose group | 31.71±3.43** |
**P<0.01 compared to control group.
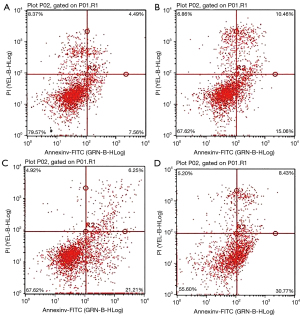
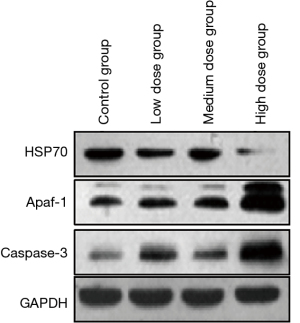
Effect of brucine on the expression of HSP70, Apaf-1, and caspase-3 proteins in PC-3 cells
The expression level of HSP70 protein in PC-3 cells in the low, medium, and high dose brucine groups was significantly lower than that in the control group (P<0.01). With the increase of dose, the expression of HSP70 protein in the PC-3 cells decreased significantly in a dose-dependent manner. Apaf-1 and caspase-3 protein expression levels in the PC-3 cells in the low, medium, and high dose brucine groups were significantly higher than those in the control group (P<0.01). With the increase of dose, the Apaf-1 and caspase-3 protein expression levels in the PC-3 cells increased significantly in a dose-dependent manner (Table 3 and Figure 3).
Table 3
| Groups | HSP70 | Apaf-1 | caspase-3 |
|---|---|---|---|
| Control group | 1.62±0.51 | 0.46±0.11 | 0.37±0.12 |
| Brucine low dose group | 1.33±0.43** | 1.24±0.24** | 0.88±0.17** |
| Brucine medium dose group | 0.99±0.41** | 1.87±0.31** | 1.35±0.24** |
| Brucine high dose group | 0.51±0.13** | 2.24±0.47** | 1.67±0.28** |
**P<0.01 compared to control group; HSP70, heat shock protein 70; Apaf-1, anti-apoptotic protease activating factor 1; caspase-3, anti-cysteine protease-3.
Discussion
The pathogenesis of prostate cancer is complex, and it may be related to a variety of factors, including genetics, endocrinology, environmental degradation, and race (6). More recently, the incidence of prostate cancer in China has been rising yearly due to the environmental and dietary changes associated with an accelerated pace of life, and prostate cancer is being diagnosed at a younger age (7). The development and exploration of an efficient and low-toxic drug to prevent and treat prostate cancer has been the focus of domestic and international research. Brucine, the main alkaloid monomer of strychnos nux-vomica, has low toxicity and has gained widespread interest in recent years in inhibiting tumor cell proliferation and inducing apoptosis in tumor cells (8). The results of this study showed that at 24 and 48 h, the activity of PC-3 cells in the low, medium, and high dose brucine groups was significantly lower than that of the control group (P<0.01). The apoptosis rate of PC-3 cells in the low, medium, and high dose brucine groups was significantly higher than that in the control group (P<0.01). It is suggested that brucine can reduce the activity of PC-3 cells and induce their apoptosis, and that brucine may present a significant prospect for the treatment of prostate cancer.
Apoptosis is an important aspect associated with the development of prostate cancer, and the induction of apoptosis is currently one of the main clinical defenses against the disease. However, the mechanisms underlying tumor cell apoptosis are complex. The death receptor signaling pathway and mitochondrial signaling pathway are considered to be the two major transduction pathways regulating tumor cell apoptosis. Among them, the mitochondrial signaling pathway was proposed in the 1990s and plays a decisive role in the apoptosis of tumor cells. When tumor cells are stimulated by radiation, chemotherapy, or drugs, the mitochondrial membrane potential decreases, and the permeability transition pore opens, which induces the release of relevant apoptosis initiators, such as caspase-3 and Apaf-1. When the mitochondrial membrane potential is completely lost, apoptosis is irreversible (9). Heat shock proteins (HSPs) have also been found to have a close relationship with the mitochondrial pathway in a study of mitochondrial transduction signaling (10). As a highly conserved protein, overexpression of HSP70 prevents the hydrogen peroxide-induced reduction in mitochondrial permeability and improves mitochondrial swelling, thus exerting an anti-apoptotic effect (11). Furthermore, it prevents the release of cytochrome C from mitochondria produced by heat or hydrogen peroxide-stimulation and binds directly to the caspase recruitment domain of Apaf-1, inhibiting the activation of some apoptotic protease factors (12). A study has also confirmed that HSP70 shows a high expression in colon, prostate, and breast cancers, and as an anti-apoptotic protein its levels are associated with tumor proliferation and clinical typing (13). Silencing the high expression of HSP70 induces apoptosis in human prostate cancer PC-3 cells. The results of this study showed that the expression level of the HSP70 protein in human prostate cancer PC-3 cells was significantly lower in the low, medium, and high dose brucine groups than in the control group. This indicates that brucine inhibited the expression of HSP70 and promoted the apoptosis of tumor cells.
Apaf-1 and caspase-3 are two essential pro-apoptotic proteins in the mitochondrial apoptosis pathway. In vertebrates, one of the main mechanisms of cellular caspase activation requires the release of cytochrome C from mitochondria and its interaction with Apaf-1. When Apaf-1 binds to cytochrome C, it further initiates the oligomerization of Apaf-1 and activates caspase-3 (14). In a previous study, HSP70 was found to bind to the amino-terminal cysteine protease recruitment domain of Apaf-1 to generate an apoptotic vesicle, which blocks the caspase-dependent apoptotic pathway and thus promotes cell proliferation (15). The results of this study showed that the expression levels of Apaf-1 and caspase-3 in PC-3 cells in the low, medium, and high dose brucine groups were significantly higher than those in the control group. Taken together, the results suggest that brucine inhibited the expression of HSP70 and prevented the production of the Apaf-1/HSP70 complex, thus promoting the expression of Apaf-1, which also further activated caspase-3 and induced the apoptosis of PC-3 cells. The molecular mechanisms of brucine inhibiting tumor include regulating the process of cell cycle, promoting apoptosis, inhibiting the ability of tumor invasion and metastasis, inducing autophagy, reversing multidrug resistance and regulating the metabolic level of tumor cells Through the exploration of the action mechanism of brucine and further research on this basis, we can constantly reveal the signal network and gene regulation sites involved in Brucine, so as to find more accurate tumor treatment targets, and provide beneficial enlightenment for the research and development of new drugs and clinical application of tumors
Conclusions
In summary, brucine downregulated HSP70 expression in human prostate cancer PC-3 cells and inhibited the mitochondrial apoptotic signaling pathway, thus exerting an anti-apoptotic effect. Brucine provides a novel perspective and potential target for the prevention and treatment of prostate cancer.
Limitation
This study lacked vivo research, which was more conducive to support the conclusion of our paper. Moreover, we did not study the lncRNA or miRNA that can regulate the brucine.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-209/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-209/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-209/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lakkis NA, Osman MH. Prostate Cancer in Lebanon: Incidence, Temporal Trends, and Comparison to Countries From Different Regions in the World. Cancer Control 2021;28:10732748211055267. [Crossref] [PubMed]
- Porcacchia AS, Pires GN, Ortiz V, et al. Prostate cancer mortality and costs of prostate surgical procedures in the Brazilian public health system. Int Braz J Urol 2022; [Epub ahead of print]. [PubMed]
- Razzaq A, Hussain G, Rasul A, et al. Strychnos nux-vomica L. seed preparation promotes functional recovery and attenuates oxidative stress in a mouse model of sciatic nerve crush injury. BMC Complement Med Ther 2020;20:181. [Crossref] [PubMed]
- Ismail TA, Shehata TM, Mohamed DI, et al. Quality by Design for Development, Optimization and Characterization of Brucine Ethosomal Gel for Skin Cancer Delivery. Molecules 2021;26:3454. [Crossref] [PubMed]
- Xu MR, Wei PF, Suo MZ, et al. Brucine Suppresses Vasculogenic Mimicry in Human Triple-Negative Breast Cancer Cell Line MDA-MB-231. Biomed Res Int 2019;2019:6543230. [Crossref] [PubMed]
- Genetics of Prostate Cancer (PDQ(R)): Health Professional Version. PDQ Cancer Information Summaries. Bethesda (MD) 2002.
- Liu X, Yu C, Bi Y, et al. Trends and age-period-cohort effect on incidence and mortality of prostate cancer from 1990 to 2017 in China. Public Health 2019;172:70-80. [Crossref] [PubMed]
- Seshadri VD. Brucine promotes apoptosis in cervical cancer cells (ME-180) via suppression of inflammation and cell proliferation by regulating PI3K/AKT/mTOR signaling pathway. Environ Toxicol 2021;36:1841-7. [Crossref] [PubMed]
- Yang Y, Wu Y, Meng X, et al. SARS-CoV-2 membrane protein causes the mitochondrial apoptosis and pulmonary edema via targeting BOK. Cell Death Differ 2022; [Epub ahead of print]. [Crossref] [PubMed]
- Gupta SD, Gomes A, Debnath A, et al. Apoptosis induction in human leukemic cells by a novel protein Bengalin, isolated from Indian black scorpion venom: through mitochondrial pathway and inhibition of heat shock proteins. Chem Biol Interact 2010;183:293-303. [Crossref] [PubMed]
- Gan L, Liu DB, Lu HF, et al. Decreased expression of the carboxyl terminus of heat shock cognate 70 interacting protein in human gastric cancer and its clinical significance. Oncol Rep 2012;28:1392-8. [Crossref] [PubMed]
- Jemmerson R, Staskus K, Higgins L, et al. Intracellular leucine-rich alpha-2-glycoprotein-1 competes with Apaf-1 for binding cytochrome c in protecting MCF-7 breast cancer cells from apoptosis. Apoptosis 2021;26:71-82. [Crossref] [PubMed]
- Brünnert D, Langer C, Zimmermann L, et al. The heat shock protein 70 inhibitor VER155008 suppresses the expression of HSP27, HOP and HSP90beta and the androgen receptor, induces apoptosis, and attenuates prostate cancer cell growth. J Cell Biochem 2020;121:407-17. [Crossref] [PubMed]
- Zhou M, Li W. Ent-Dihydrotucumanoic acid promotes apoptosis in PC-3 human prostate cancer cells. Cell Mol Biol (Noisy-le-grand) 2019;65:114-8. [Crossref] [PubMed]
- Li Y, Pan J, Gou M. The Anti-Proliferation, Cycle Arrest and Apoptotic Inducing Activity of Peperomin E on Prostate Cancer PC-3 Cell Line. Molecules 2019;24:1472. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


