
Induction of ligustrazine apoptosis of A549 cells through activation of death receptor pathway
Introduction
The incidence and case fatality rate of lunger remain high, with many cases only being detected at an advanced stage. The unlimited proliferative capacity of tumor cells is the greatest obstacle in tumor therapy. Current research suggests that tumor cells evade radiotherapy and drug treatment through multiple pathways, one of which is cell death escape (1). A key point in drug development is the search for potential agents that can effectively block cell survival, causing apoptosis, and this particular aspect of tumor cell survival has become a major focus in oncology. Apoptosis is an important mechanism in maintaining the internal ordered activity of living bodies generally under specific conditions through the activation, expression, and regulation of a series of genes that are spontaneously ordered to maintain normal metabolism (2). Disruption of this balance can lead to lesions such as tumors. Among the cell signaling pathways, the proapoptotic and antiapoptotic signaling nodes are the locations of selected targets in therapeutic studies of tumors and often serve as detection points for tumor initiation, progression, and therapeutic efficacy (3,4).
Chuanxiong is a traditional Chinese medicine that is commonly used in the treatment of cardiovascular diseases. Intensive study found ligustrazine, an extract of Ligusticum chuanxiong Hort, to be one of the active ingredients that has a significant effect on cytokines, such as tumor necrosis factor and interferon. Generally, studies on this subject indicate that the pathways involved in the treatment of cardiovascular diseases overlap considerably with those pathways involved in the emergence and development of tumors. This preliminary study in lung cancer treatment examined the ligustrazine-induced apoptosis of tumor cells via the activation of death receptors.
We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-455/rc).
Methods
Reagents and equipment
For the reagents and equipment used, an MTT kit was purchased form Promega (Madison, WI, USA); Annexin V-FITC/PI Kit, Fas antibody, and Fas-L antibody with FITC label were purchased form BD Biosciences (San Jose, CA, USA); qPCR SuperMIX was purchased form Toyobo (Osaka, Japan); caspase 8 and caspase 3 enzyme-linked immunosorbent assay (ELISA) kits were purchased form RayBiotech (Peachtree Corners, GA, USA); and ligustrazine was purchased form Chengdu Herbpurify (Chengdu, China). Flow cytometry was provided BD Biosciences, gene amplification instruments were obtained from Bio-Rad Laboratories (Hercules, CA, USA), and a microplate reader was acquired from BioTek (Winooski, VT, USA).
Cell culture and viability assay
Human lung cancer cell line A549 [American Type Culture Collection (ATCC); CCL-185, Manassas, VA, USA] was cultured with RPMI1640 (Gibco, Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco) in a 37 ℃, 5% CO2 atmosphere. The gradient concentration of 0, 1, 2, 4, 8, and 16 mg/mL medium with ligustrazine was added to a 96-well plate, with the cell concentration being 5×103/well. The MTT kit was used to detect the cell survival rate after 24 hours.
Apoptosis analysis
Three ligustrazine concentrations without cytotoxicity were selected for all subsequent experiments. The cell concentration was adjusted to 5×104/mL in a 35-mm cell culture dish. After 24 hours of culture with ligustrazine, the cells were washed with PBS for 2 times. Subsequently, the cells were resuspended with binding buffer 100 µL and were incubated in the dark with annexin V/PI for 20 min at room temperature. Then apoptosis was analyzed by flow cytometry.
qRT-PCR
The cell was treated in same fashion detailed in section Apoptosis analysis. Total RNA was extracted from cultured cells using RNeasy Mini QIAcube Kit (QIAGEN, Hilden, Germany). Quantitative reverse transcription PCR (qRT-PCR) was performed with a SYBR Green Realtime PCR Master Mix (Toyobo). The primer sequences were purchased from Sangon Biotech (Shanghai, China), and the primer sequences are listed in Table 1.
Table 1
| Gene | Forward primer (5’→3’) | Reverse primer (5’→3’) |
|---|---|---|
| Fas | GAAGAGACCCCTGTGGTATTTGA | ACACTTTTCCGCTCACAATCAGA |
| Fas-L | TGTTAAATGGGCCACTTTCCTC | GGATGTTTCAGCTCTTCCACCTAC |
| TRAIL | CTGATCGTGATCTTCACAGTGCTC | CAGCAGGGTAGACTCAGCAAGG |
| Caspase 8 | CAAGAGGAAATCTCCAAATGCAAAC | CAGGATGTCCAACTTTCCTTCTCC |
| Caspase 3 | GTGAAGAGTTGGACCACCATAGCA | AATGAGTCAACCAAGTTCGCACAC |
| β-Actin | TGGCACCCAGCACAATGAA | CTAAGTCATAGTCCGCCTAGAAGC |
Expression of Fas and Fas-L
To determine the expression of Fas and Fas-L, the A549 cells were treated in the same manner as described in section Apoptosis analysis. After being washed with phosphate-buffered saline (PBS) 3 times, the cells were adjusted to 3×105/mL and incubated with Fas and Fas-L antibody with FITC label in the dark for 20 min at room temperature. The cells were analyzed by flow cytometry
Determination of caspase 8 and caspase 3 activity
To determine caspase 8 and caspase 3 activity, the A549 cells were treated in the same manner as described in section Apoptosis analysis. With 6-well plates, caspase 8 and caspase 3 kits were used for activity analysis according to the manufacturer’s instructions.
Statistical analysis
Data for independent experiments were presented as means ± standard error of the mean (SEM). Normally distributed data were compared by unpaired Student’s t-test for two groups comparisons and one-way analysis of variance (ANOVA). P values <0.05 were considered significant difference. Data statistically analysis were using GraphPad Prism 5.0 (GraphPad Software., San Diego, CA, USA) and SPSS software, version 23.0 (IBM Corp., Armonk, NY, USA). All biological replicates were performed at least three times. Experiment and analysis were bi-blinding.
Results
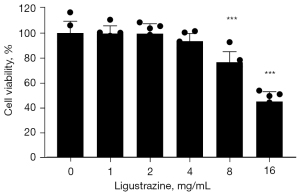
Ligustrazine inhibited A549 cell proliferation
Ligustrazine showed an ability to inhibit A549 cells proliferation in a dose-dependent manner. Significant cytotoxicity appeared at a dose of more than 8 mg/mL (P<0.001). Consequently, 1, 2, and 4 mg/mL doses were selected for the ensuing experiments (Figure 1).
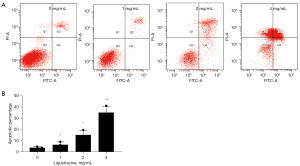
Flow cytometry analysis indicated ligustrazine could promote A549 cell apoptosis
Flow cytometry indicated that, compared with the control, ligustrazine significantly increased the percentage of apoptosis in a dose-dependent manner. With reference to the control group, the differences between the groups in each concentration were statistically significant (Figure 2).
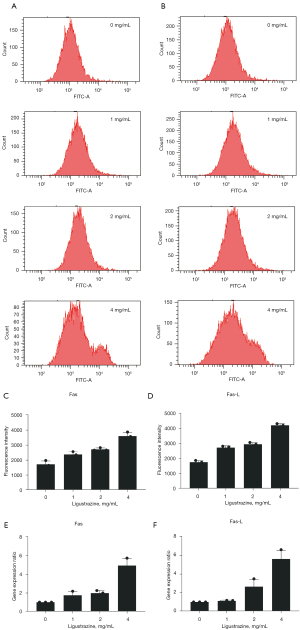
Ligustrazine promoted Fas and Fas-L expression in A549 cells
After treatment with ligustrazine, the gene and protein expressions of Fas and Fas-L all increased in a dose-dependent manner, with significant differences compared with the control group. Realtime PCR assays showed significant upregulation of Fas gene expression in the 1, 2, and 4 mg/mL groups and of Fas-L gene expression in the 2 and 4 mg/mL groups. Flow cytometry was used to determine Fas and Fas-L expression on the cell surface and revealed a significant difference when the cells were exposed to lower doses (1 and 2 mg/mL), with particularly significant increases at higher doses (4 mg/mL). However, the 1 and 2 mg/mL groups did not significantly differ from each other. The results of gene level and protein level detection were consistent (Figure 3).
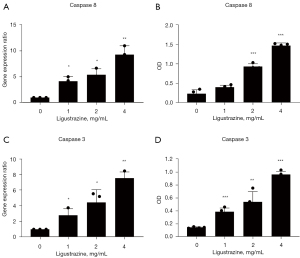
Ligustrazine promoted caspase 8 and caspase 3 expression in A549 cells
After treatment with ligustrazine, the gene and protein expressions of the caspase 8 and caspase 3 groups appeared to be significantly different from those of the control group, with this difference being dose-dependent. Realtime PCR assay showed the gene expression of caspase 8 and caspase 3 to be significantly upregulated in each group after drug administration. ELISA assays showed that the protein expression of caspase 8 and caspase 3 was significantly upregulated compared with the control group, with the 4 mg/mL dose yielding a highly significant difference. The results of gene level and protein level detection were consistent (Figure 4).
Discussion
The apoptosis usually featured by specific morphological and energy-dependent events. Apoptosis was believed to involve in a serious progress events: cell transformation, immune system development and function, atrophy, development of embryonic, and cell death. Abnormal apoptosis resulted in various diseases, including neurodegenerative diseases, ischemic cascade, cancers and autoimmune diseases. Apoptosis was believed has great potential for its ability to regulate cell death (5).
Apoptosis could be induced by lots of stimuli and conditions, even the drugs of cancer could cause DNA damage, and finally resulted in apoptosis by p53 pathway (6). Corticosteroids (a kind of hormone) can lead to apoptosis in thymocytes cells.
Various low doses noxious stimuli such as heat, radiation, hypoxia, and cancer drugs can lead to apoptosis, while higher concentration of them even cause necrosis. Recent studies demonstrated Chinese also regulated cell apoptosis such as baicalein increased cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-κB pathway (7), “Shenqi San” inhibited proliferation of A549 cells via inducing apoptosis (8), lotus leaf flavonoids induce apoptosis via ROS/p38 MAPK pathway (9), baicalin inhibited the proliferation and migration of A549 and H1299 by activating the SIRT1/AMPK signaling pathway (10), gracillin isolated from reineckia carnea induced apoptosis of A549 cells via the mitochondrial pathway (11). Besides, there were other moleculars reported regulated A549 apoptosis such as Cyclophilin A regulates the apoptosis of A549 cells by stabilizing Twist1 protein (12), circRNA (HSA_CIRC_0004050) regulated proliferation and apoptosis of A549 cells through ERK/JNK signaling pathway (13).
Up to now, apoptosis was divided into extrinsic and intrinsic cell death. Extrinsic apoptosis was triggered on specific cell membrane receptor while the intrinsic apoptosis was induced by mitochondrial pathway. However, recent study revealed that the two pathways were intercrossed, some molecules could influence others in the other pathways (14). Another apoptosis pathway could triggered by T cell-mediated cytotoxicity and perforin granzyme. It can be induced by granzyme A or B. The granzyme A activated a caspase-independent pathway by DNA single-stranded damage (15).
The results of the present study indicated that ligustrazine promoted apoptosis in the human lung cancer cell line A549. This facilitatory capacity appeared to be exerted through the Fas pathway. In flow cytometry detection, it was found that the Fas and Fas-L expression on the surface of A549 cells was positively correlated with the concentration of ligustrazine. Fas is a key point in the apoptotic process, as it provides strong support for the anti-lung cancer effect of ligustrazine. Uncontrolled proliferation and malfunctioning apoptosis are the biological features that distinguish tumor cells from normal cells (16). Thus, exploring ways to promote apoptosis is an effective way to treat cancer disease.
Apoptosis is a type of physiological death whose occurrence is critical in maintaining homeostasis. Gene regulation in apoptosis is involved in numerous signaling pathways. Fas and its ligand, Fas-L, are membrane surface molecules closely related to apoptosis, and the Fas/Fas-L system is integral to mediating apoptosis and has been primarily used to study tumor therapy (17,18). Both vivo and in vitro studies have demonstrated that Fas mAb can bind to Fas, resulting in the death of cells expressing Fas. Thus Fas, as a target, is considered a key factor in cell death (19). High expression of Fas on the cell membrane is thus highly indicative of the susceptibility of Fas signaling pathway to induce apoptosis; however, the cutoff value between tumor cells is not uniform. Studies have shown that several molecules including Bcl-2 family, c-myc, Ras, and Fap-1 may participate in Fas-mediated apoptosis (18,20-23). The death of cells with a surface expression of Fas molecules may be induced by T lymphocytes positive for Fas-L (24). Therefore, a drug that can promote Fas expression in tumor cells may be effective in treating tumors and thus be worthy of more in-depth study.
The present experimental results demonstrated that ligustrazine exhibited an obvious inhibitory effect on the human lung cancer cell line A549. Furthermore, as detected by qRT-PCR and flow cytometry, ligustrazine was found to upregulate Fas gene expression of lung cancer cells A549 and realize successful gene upregulation. Indeed ligustrazine elevates Fas expression on the surface of lung cancer cell line A549, which is helpful to enhancing the mutual recognition of Fas and Fas-L, triggering the cell interior to initiate the apoptotic program, and inducing the cell to undergo programmed death.
Moreover, our experiment confirmed that ligustrazine enhanced expression of caspase 8 and caspase 3. It can thus be deduced that upregulated Fas can promote the apoptosis of A549 cells via caspase pathways (25,26). In Fas-L-mediated apoptosis, caspase 8 acts as a key initiator, which, when activated, will trigger a caspase proteolysis cascade and thus activate apoptotic behavior (27,28). After caspase 8 activation by Fas, a cascade of signaling via the caspase pathway is initiated, which leads to caspase 3 activation (29). Being capable of inducing apoptosis, caspase 3 has a specific hallmark role in the caspase pathway. The activation of caspase 3 is a sign that apoptosis has entered an irreversible stage (30). Ligustrazine promotes the activation of caspase 3, which can inhibit tumor cell propagation and metastatic ability (31,32). In our study, Fas activated caspase 8 and promoted the initiation of apoptosis, which in turn triggered caspase 3 expression and regulated the caspase pathway to promote apoptosis in A549 cells.
The extrinsic apoptosis pathway which could be triggered by extracellular signals. Generally, cell death signals bind to TNFdeath receptors (33). Death ligands include Fas ligand (Fas-L), tumor necrosis factor (TNF) and TNF-related apoptosis-inducing ligand (TRAIL). The initiator procaspases-8 and procaspases-10 bind to the adaptor protein and form the death-inducing signaling complex (DISK) (34), and activated by DISK. Then the caspases-3, -6, and -7 begin to cleave proteins and cytoskeleton, which finally leads to apoptosis (35).
The re-expression of apoptotic molecules, which resensitizes cells to apoptosis induction and further promotes apoptosis, has become an important idea and theory for tumor therapy (36).
Conclusions
In this study, ligustrazine was evaluated in regard to the treatment of lung cancer through the induction of apoptosis, and obvious effects were detected throughout the course of the experiment. Further studies on ligustrazine will use Fas-related signals combined with pharmacodynamics to provide a technical basis for developing potential drug candidates with low toxicity and high activity for lung cancer treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-455/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-455/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-455/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cha JH, Chan LC, Li CW, et al. Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell 2019;76:359-70. [Crossref] [PubMed]
- Matsuura K, Canfield K, Feng W, et al. Metabolic Regulation of Apoptosis in Cancer. Int Rev Cell Mol Biol 2016;327:43-87. [Crossref] [PubMed]
- Gong P, Wang Y, Jing Y. Apoptosis Induction byHistone Deacetylase Inhibitors in Cancer Cells: Role of Ku70. Int J Mol Sci 2019;20:1601. [Crossref] [PubMed]
- Tunjung WAS, Sayekti PR. Apoptosis induction on human breast cancer T47D cell line by extracts of Ancorina sp. F1000Res 2019;8:168. [Crossref] [PubMed]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495-516. [Crossref] [PubMed]
- Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol 2001;41:367-401. [Crossref] [PubMed]
- Yu M, Qi B, Xiaoxiang W, et al. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-kappaB pathway. Biomed Pharmacother 2017;90:677-85. [Crossref] [PubMed]
- Xia Y, Shi L, Ai ZZ, et al. Chinese medicine formula "Shenqi San" extract inhibits proliferation of human lung adenocarcinoma A549 cells via inducing apoptosis. J Huazhong Univ Sci Technolog Med Sci 2017;37:766-71. [PubMed]
- Jia XB, Zhang Q, Xu L, et al. Lotus leaf flavonoids induce apoptosis of human lung cancer A549 cells through the ROS/p38 MAPK pathway. Biol Res 2021;54:7. [Crossref] [PubMed]
- You J, Cheng J, Yu B, et al. Baicalin, a Chinese Herbal Medicine, Inhibits the Proliferation and Migration of Human Non-Small Cell Lung Carcinoma (NSCLC) Cells, A549 and H1299, by Activating the SIRT1/AMPK Signaling Pathway. Med Sci Monit 2018;24:2126-33. [Crossref] [PubMed]
- Yang J, Cao L, Li Y, et al. Gracillin Isolated from Reineckia carnea Induces Apoptosis of A549 Cells via the Mitochondrial Pathway. Drug Des Devel Ther 2021;15:233-43. [Crossref] [PubMed]
- Wu Y, Ma Z, Zhang Y, et al. Cyclophilin A regulates the apoptosis of A549 cells by stabilizing Twist1 protein. J Cell Sci 2022;135:jcs259018. [Crossref] [PubMed]
- Wang Y, Zang RK, Du YN. HSA_CIRC_0004050 on proliferation and apoptosis of A549 cells through ERK/JNK signaling pathway. J Biol Regul Homeost Agents 2020;34:2037-47. [PubMed]
- Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2002;2:277-88. [Crossref] [PubMed]
- Martinvalet D, Zhu P, Lieberman J. Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity 2005;22:355-70. [Crossref] [PubMed]
- Sack LM, Davoli T, Li MZ, et al. Profound Tissue Specificity in Proliferation Control Underlies Cancer Drivers and Aneuploidy Patterns. Cell 2018;173:499-514.e23. [Crossref] [PubMed]
- Solodeev I, Meilik B, Volovitz I, et al. Fas-L promotes the stem cell potency of adipose-derived mesenchymal cells. Cell Death Dis 2018;9:695. [Crossref] [PubMed]
- Michita RT, Zambra FMB, Fraga LR, et al. The role of FAS, FAS-L, BAX, and BCL-2 gene polymorphisms in determining susceptibility to unexplained recurrent pregnancy loss. J Assist Reprod Genet 2019;36:995-1002. [Crossref] [PubMed]
- Tang H, Zhang S, Huang C, et al. MiR-448-5p/VEGFA Axis Protects Cardiomyocytes from Hypoxia Through Regulating the FAS/FAS-L Signaling Pathway. Int Heart J 2021;62:647-57. [Crossref] [PubMed]
- Xu D, Wang B, Chen PP, et al. c-Myc promotes tubular cell apoptosis in ischemia-reperfusion-induced renal injury by negatively regulating c-FLIP and enhancing FasL/Fas-mediated apoptosis pathway. Acta Pharmacol Sin 2019;40:1058-66. [Crossref] [PubMed]
- Ren X, Zhang Z, Tian J, et al. The downregulation of c-Myc and its target gene hTERT is associated with the antiproliferative effects of baicalin on HL-60 cells. Oncol Lett 2017;14:6833-40. [Crossref] [PubMed]
- Dogru G, Ay OI, Erdal ME, et al. The role of certain gene polymorphisms involved in the apoptotic pathways in polycythemia vera and essential thrombocytosis. Adv Clin Exp Med 2017;26:761-5. [Crossref] [PubMed]
- Xiao Y, Liu T, Liu X, et al. Total Astragalus saponins attenuates CVB3-induced viral myocarditis through inhibiting expression of tumor necrosis factor alpha and Fas ligand. Cardiovasc Diagn Ther 2019;9:337-45. [Crossref] [PubMed]
- Zhu J, Petit PF, Van den Eynde BJ. Apoptosis of tumor-infiltrating T lymphocytes: a new immune checkpoint mechanism. Cancer Immunol Immunother 2019;68:835-47. [Crossref] [PubMed]
- Sekiguchi Y, Yamada M, Noguchi T, et al. The anti-cancer drug gefitinib accelerates Fas-mediated apoptosis by enhancing caspase-8 activation in cancer cells. J Toxicol Sci 2019;44:435-40. [Crossref] [PubMed]
- Jung KT, Oh SH. Polyubiquitination of p62/SQSTM1 is a prerequisite for Fas/CD95 aggregation to promote caspase-dependent apoptosis in cadmium-exposed mouse monocyte RAW264.7 cells. Sci Rep 2019;9:12240. [Crossref] [PubMed]
- Henry CM, Martin SJ. Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory "FADDosome" Complex upon TRAIL Stimulation. Mol Cell 2017;65:715-729.e5. [Crossref] [PubMed]
- Du P, Li SJ, Ojcius DM, et al. A novel Fas-binding outer membrane protein and lipopolysaccharide of Leptospira interrogans induce macrophage apoptosis through the Fas/FasL-caspase-8/-3 pathway. Emerg Microbes Infect 2018;7:135. [Crossref] [PubMed]
- Raptis V, Bakogiannis C, Loutradis C, et al. Serum Fas Ligand, Serum Myostatin and Urine TGF-beta1 Are Elevated in Autosomal Dominant Polycystic Kidney Disease Patients with Impaired and Preserved Renal Function. Kidney Blood Press Res 2018;43:744-54. [Crossref] [PubMed]
- Rong Y, Liu W, Wang J, et al. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis 2019;10:340. [Crossref] [PubMed]
- Muscari I, Adorisio S, Liberati AM, et al. Bcl-xL overexpression decreases GILZ levels and inhibits glucocorticoid-induced activation of caspase-8 and caspase-3 in mouse thymocytes. J Transl Autoimmun 2020;3:100035. [Crossref] [PubMed]
- García-Argüello SF, Lopez-Lorenzo B, Cornelissen B, et al. Development of [18F]ICMT-11 for Imaging Caspase-3/7 Activity during Therapy-Induced Apoptosis. Cancers (Basel) 2020;12:2191. [Crossref] [PubMed]
- Goldar S, Khaniani MS, Derakhshan SM, et al. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev 2015;16:2129-44. [Crossref] [PubMed]
- Liu H, Su D, Zhang J, et al. Improvement of Pharmacokinetic Profile of TRAIL via Trimer-Tag Enhances its Antitumor Activity in vivo. Sci Rep 2017;7:8953. [Crossref] [PubMed]
- Zaman S, Wang R, Gandhi V. Targeting the apoptosis pathway in hematologic malignancies. Leuk Lymphoma 2014;55:1980-92. [Crossref] [PubMed]
- Rao S, Mondragón L, Pranjic B, et al. AIF-regulated oxidative phosphorylation supports lung cancer development. Cell Res 2019;29:579-91. [Crossref] [PubMed]


