
Dual inhibition of autophagy and PI3K/mTOR pathway as a potential therapeutic strategy against laryngeal squamous cell carcinoma
Introduction
Laryngeal squamous cell carcinoma (LSCC) is the second most prevalent head and neck squamous cell carcinoma (HNSCC) throughout the world, and its incidence has significantly increased in the past decade (1). Despite advances in the treatments, 5-year survival rate of LSCC has decreased from 66% to 63% over the past 40 years (2). This may be related to the popularization of the concept of preserving laryngeal function during this period. In China, approximately 40% of LSCC patients are diagnosed at an advanced stage and total laryngectomy is usually required according to the National Comprehensive Cancer Network (NCCN) treatment guidelines (3). For patients with LSCC, radical surgical excision is of paramount importance for achieving a favorable prognosis, but it inevitably affects their vocalization and swallowing to a great extent. Considering that the postoperative life quality and overall survival rate of patients with LSCC remain unsatisfactory, new chemotherapy strategies or targeted therapy are urgently required (4).
The mammalian target of rapamycin (mTOR) is overactivated in many malignancies and plays a crucial part in PI3K/Akt/mTOR pathway (5), which can promote cell growth and proliferation. Moreover, PI3K/Akt/mTOR pathway seemed to be a prognostic factor and a predictor of response to radiotherapy and chemotherapy in clinical practice (6). Since its critical influence on tumor activity, regulation of this signaling pathway has become a potential target for LSCC intervention.
Furthermore, recent studies have indicated that autophagy inhibition could enhance anticancer therapies in various malignancies (7,8), suggesting that autophagy is also emerging as a promising therapeutic target in oncotherapy. Considering that the PI3K/Akt/mTOR pathway is also involved in the regulation of autophagy, along with the possible limitation of monotherapy with PI3K or mTOR inhibitor, a combination of dual PI3K/mTOR inhibitor and autophagy inhibitor has become a novel research direction.
To identify a potential therapy strategy for LSCC, we compared the effects of three PI3K/mTOR signaling pathway inhibitors (LY294002, rapamycin, NVP-BEZ235) against LSCC cells in vitro, and the synergistic effect of the combined application of dual PI3K/mTOR inhibitor and autophagy inhibitor was evaluated in vivo. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2325/rc).
Methods
Cell lines
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Two LSCC cell lines, AMC-HN-8 and Hep-2, were acquired from the Stem Cell Bank of the Chinese Academy of Science. Both cells were cultured in RPMI-1640 medium (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY), 1% penicillin-streptomycin (Genom Biotechnology, China), and maintained in 5% CO2 at 37 ℃. In this experiment, cells were cultured 24 hours in advance and underwent subsequent treatment.
Reagents
Antibodies against caspase-8 (SAB5701263), caspase-3 (SAB5700914), caspase-9 (SAB5700785), cyclin-D1 (SAB140551), cyclin-E2 (SAB4503557), phospho-Akt (05-1003), Akt (SAB4500797), phospho-P70S6K (SAB4301562), P70S6K (SAB2500736), phospho-4E-BP1 (SAB4301339), 4E-BP1 (SAB4500736), phospho-S6RP (SAB5700395), S6RP (SAB4300484), LC3-II (L8918), LC3-I (L8918), and GAPDH (G9545) were purchased from Sigma-Aldrich, USA. PI3K/mTOR inhibitors LY294002 (S1105), rapamycin (S1039), NVP-BEZ235 (S1009), and autophagy inhibitors 3-Methyladenine (3-MA, S2767) and Chloroquine Phosphate (CQ, S4157) were purchased from Selleck, USA.
MTT detection for the calculation of drug IC50
Hep-2 and AMC-HN-8 cells were cultured in 1640 medium containing 10% FBS until the cells grew to 80–90% and digested with trypsinization. The cell suspension concentration was adjusted for 10,000 cells per well in 96-well plates. After cultured for 24 hours, cells were treated with different concentrations of LY294002 (1, 5, 10, 20, 40, 80, 160 µM), rapamycin (1, 5, 10, 20, 40, 80, 160 µM), and NVP-BEZ235 (0.1, 0.5, 1, 2, 4, 8, 16 µM) for 72 h. DMSO served as the control group. The culture medium was not replaced during the period. Twenty microliters of MTT (5 mg/mL) were added to each well later, and all plates were further incubated for 4 h. Later, 150 µL of DMSO was added, and the OD490 values were measured. The inhibition rate was selected as the y-coordinate and the drug concentration (lnX) as the x-coordinate. The fitting curve formula Y = A * lnX + B was used to calculate the semi-inhibition rate of cells, IC50 = exp [(0.5 − b)/a]. All assays were performed in three replicates, and each experiment was repeated three times.
Western blotting
Hep-2 and AMC-HN-8 cells were cultured in 1640 medium containing 10% FBS until the number of cells reached 5×106–5×107. Hep-2 cells were treated with or without LY294002 (7.5 µM), rapamycin (15 nM), and NVP-BEZ235 (1.5 µM), while AMC-HN-8 cells were treated with or without LY294002 (6 µM), rapamycin (15 nM), and NVP-BEZ235 (0.75 µM). The culture medium was not replaced for 72 hours. Whole cell protein lysates were obtained later using RIPA lysis buffer solution (Beyotime, China). Equivalent quantities of cell lysate protein (25 µg) were exposed to SDS-PAGE and transferred to a PVDF membrane (Millipore, USA), which was blocked with 5% nonfat milk for 1 hour at room temperature and incubated with antibodies at 4 ℃ overnight. Dilutions of the primary antibodies are as follows: caspase-8 (1:500), caspase-3 (1:500), caspase-9 (1:500), cyclin-D1 (1:1,000), cyclin-E2 (1:1,000), phospho-Akt (1:500), Akt (1:500), phospho-P70S6K (1:500), P70S6K (1:500), phospho-4E-BP1 (1:500), 4E-BP1 (1:500), phospho-S6RP (1:500), S6RP (1:500), LC3-II (1:500), LC3-I (1:500), and GAPDH (1:1,000). Dilution of the secondary antibodies was 1:5000. Diluted primary antibodies were generally repeated no more than three times, while the secondary antibodies were used only once. The protein bands were visualized by using an enhanced chemiluminescent substrate (Millipore, USA). ImageJ software was used to analyze relative protein levels and normalize the internal reference protein GAPDH.
Apoptosis assay
An Annexin V-FITC/PI Detection Kit (Invitrogen, USA) was used to examine cell apoptosis according to the manufacturer’s instructions. Referring to the above-described processing method, the treated LSCC cells were collected and stained with Annexin V-FITC and PI. After 30 min of incubation in the dark, the cell apoptosis ratio was detected using FCM (Becton Dickinson, USA).
The TUNEL kit (Roche, Switzerland) was used to detect apoptotic cells in tumors according to the manufacturer’s instructions. The proportion of TUNEL-positive cells showing a brown color on each section was counted to determine the occurrence of apoptosis.
Cell cycle assay
The cell cycle was examined using the propidium iodide (PI) technique. After treatment with LY294002, rapamycin, or NVP-BEZ235 for 72 h without a replacement of culture medium, the LSCC cells were starved for 24 h in RPMI-1640 without serum and the above inhibitors. Single cell suspensions were then fixed in 75% ethanol overnight at −20 ℃, washed twice with PBS, and incubated with PI/RNase staining buffer (BD Biosciences). Samples were tested on a flow cytometer (BD Biosciences) and analyzed with FlowJo software (FlowJo LLC).
Invasion and migration assay with a trans-well chamber
The invasive and migratory capabilities of Hep-2 and AMC-HN-8 cells following treatment with the three inhibitors were evaluated by trans-well assay. For invasion, 100 µL Matrigel (Becton Dickinson, USA) was added to the bottom of each upper chamber in advance and dried for 4 h at 37 ℃ to a gelatinous form. Then, for both experiments, 5×105/mL cells were placed in the upper chambers with LY294002, rapamycin, NVP-BEZ235, DMSO separately, and incubated for 24 h at 37 ℃. The lower chambers were filled with a culture medium with 600 µL of 20% FBS. The culture medium was not replaced during the period. Cells in the chambers were later fixed with 4% paraformaldehyde and stained with 0.1% crystal violet (Beyotime, China). Five random fields were selected to count, and the results were averaged and plotted.
ATG7 siRNA Interference
We transfected cells with siRNA oligos against ATG7 with the purpose of genetically inhibit autophagy. The sequences of ATG7 were as follows: Atg7-1 5'-GATCCGGTGCTGGTTTCCTTGCTTAACTCGAGTTAAGCAAGGAAACCAGCACCTTTTTG-3' and antisense 5'-AATTCAAAAAGGTGCTGGTTTCCTTGCTTAACTCGAGTTAAGCAAGGAAACCAGCACCG-3'; Atg7-2 5'-GATCCGCAGCCTCTCTATGAGTTTGACTCGAGTCAAACTCATAGAGAGGCTGCTTTTTG-3' and antisense 5'-AATTCAAAAAGCAGCCTCTCTATGAGTTTGACTCGAGTCAAACTCATAGAGAGGCTGCG-3'.
A total of 1×105 AMC-HN-8 and Hep-2 cells were seeded in 6-well plates overnight before transfection. Cells were transfected with siRNA oligonucleotides at 4 µg/250 µL medium with 10 µL Lipofectamine 2000 (Invitrogen, USA) each well according to the manufacturer’s instructions.
Tumorigenicity assay in nude mice
Experiments were performed under a project license (No. KJ2008-01) granted by Ethics Committee of the EENT Hospital, Fudan University, in compliance with Fudan University guidelines for the care and use of animals. All male 6- to 8-week-old BALB/c nude mice (SLAC Laboratory Animal Co., Ltd, Shanghai) were inoculated with 6×106 Hep-2 cells before being randomly divided into four groups (n=5 each) as follows: (I) control group; (II) NVP-BEZ235 group, oral administration of NVP-BEZ235 (30 mg/kg/d); (III) CQ group, intraperitoneal (i.p.) administration of CQ (50 mg/kg/d); (IV) NVP-BEZ235+CQ group, mice were treated with NVP-BEZ235 (30 mg/kg/d, p.o.) and CQ (50 mg/kg/d, i.p.) simultaneously. The tumor sizes were measured once a week with calipers. All surviving mice were sacrificed 28 days later to collect tumors for further detection. Tumor volumes were calculated: tumor volume = (4π/3) × (width/2)2× (length/2). Tumor cell apoptosis of each group were analyzed.
Statistical analysis
Data analysis was accomplished using SPSS 22.0 software. Differences between groups were assessed by the Student’s t-test (two-tailed), and the results are shown as means ± SEM. P<0.05 was considered statistically significant.
Results
Dual PI3K/mTOR inhibitor NVP-BEZ235 leads to cell cycle arrest and apoptosis in LSCC cell lines
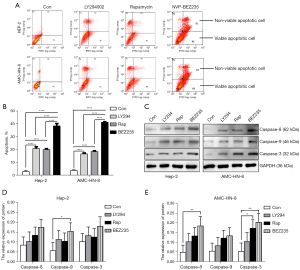
To clarify the role of PI3K/Akt/mTOR pathway in LSCC, Hep-2 and AMC-HN-8 cell lines were treated with PI3K inhibitor LY294002, mTOR inhibitor rapamycin, and dual PI3K/mTOR inhibitor NVP-BEZ235 separately. Different drug concentrations were applied to two LSCC cell lines according to the results of MTT assay (Figure S1). Cell apoptosis and the cell cycle were then assessed. NVP-BEZ235 elicited significant blockage at G2/M phase, and the protein levels of cyclin D1 and cyclin E2 also decreased in both cell lines than LY294002 and rapamycin (Figure 1), which suggested its better effect on the cell cycle as a dual PI3K/mTOR inhibitor. Treatment with all three inhibitors resulted in significant apoptosis in Hep-2 and AMC-HN-8, especially with NVP-BEZ235 (Figure 2A,2B), whereas the protein level of caspases 3, 8, and 9 increased after inhibitor treatment (Figure 2C-2E).
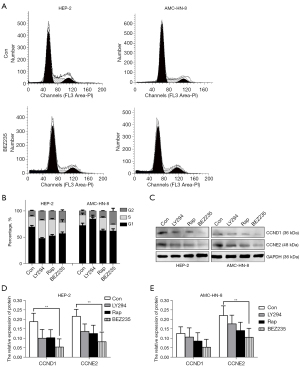
NVP-BEZ235 shows more potent and dual blocking effects for mTORC1 and mTORC2
We next tested the activation of AKT, p70S6K, 4EBP1, and S6RP in the two cell lines after the treatment with the inhibitors. NVP-BEZ235 modulated not only mTORC1 targets p-70S6K, p-4EBP1 and p-S6RP, but also mTORC2 targets p-Akt (Figure 3). To evaluate antitumor effects, we further manipulated the migration and invasion test by transwell chamber assay in Hep-2 and AMC-HN-8 treated with Ly294002, rapamycin, NVP-BEZ235, or DMSO (as control). All three inhibitors reduced cell migration and invasion in vitro (Figure 4); however, NVP-BEZ235 elicited the most significant effect.
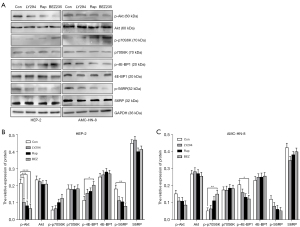
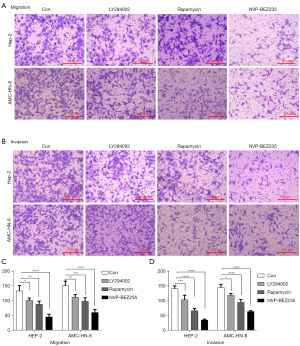
Considering the above assessment, NVP-BEZ235 had a better block effect on PI3K/Akt/mTOR pathway as a dual PI3K/mTOR inhibitor on both LSCC cell lines than LY294002 and rapamycin.
Synergic antitumor effect of combination NVP-BEZ235 with autophagy inhibition in vitro
As NVP-BEZ235 can regulate cell autophagy in many tumors (9,10), we investigated the regulatory effects of all three inhibitors on autophagy in LSCC cell lines. Autophagy control was observed using a confocal laser scanning microscope after acridine orange staining (Figure S2) and verified by LC3, a protein embedded in autophagosomes that became lipids during active autophagy. LC3II/LC3I ratio was obtained using western blotting analysis (Figure 5). According to the analyses, the trend of LC3I and LC3II change was consistent in the two cells. Although some of the changes were not statistically significant, the rise of LC3II/LC3I ratio in both cell lines suggested that NVP-BEZ235 is more effective in promoting autophagy.
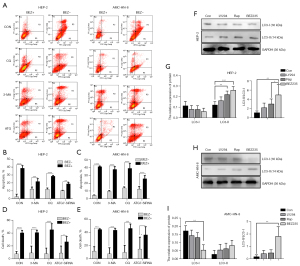
Furthermore, we tested the combination of NVP-BEZ235 with autophagy inhibitors, 3-methyladenine (3-MA), chloroquine (CQ) and ATG7-SiRNA. 3-MA inhibits the initiation of autophagy, CQ inhibits the fusion of autophagosomes and lysosomes; and ATG7-SiRNA, is a genetic silencing of Atg7, which inhibits the early phase of autophagy. The results showed that cell death was significantly increased when NVP-BEZ235 was combined with autophagy inhibitors (Figure 5), although LC3II/LC3I ratio and the expression level of caspase 3 were not significantly changed by the combination. This result strongly indicates that the combination of NVP-BEZ235 and autophagy inhibitors has a synergic antitumor effect through increasing LSCC cell apoptosis and death.
Synergistic antitumor effect of combination NVP-BEZ235 with autophagy inhibition in vivo
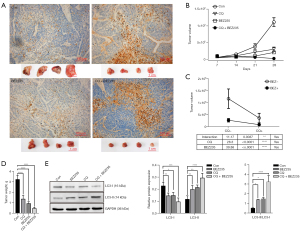
We then tested the antitumor efficacy of the combination of NVP-BEZ235 with CQ in vivo. BALB/c mice bearing Hep-2 xenograft tumors were treated with NVP-BEZ235, CQ, NVP-BEZ235+CQ, and physiological saline solution as a control for 4 weeks after tumor cell injection. First, we tested the drug toxicity of all groups by evaluating the changes in animal weight, behavior, and appearance. The tumor size was then measured weekly before the animals were sacrificed. The final tumor masses were dissected after the last measurement. Combination of NVP-BEZ235 and CQ demonstrated synergistic effect against autophagy, indicated by reduced tumor size and final tumor weight (Figure 6). The interaction effect was elicited by the interaction index (P<0.0001). Based on TUNEL assay of fragmented DNA of apoptotic cells in final tumor masses, the most apoptotic cells were found in the group treated with NVP-BEZ235 and CQ.
Discussion
In this study, the dual PI3K/mTOR inhibitor NVP-BEZ235 revealed a better effect on the cell cycle, cell apoptosis, migration, and invasion in LSCC. The expression changes in downstream molecules of PI3K/Akt/mTOR signaling pathway further explained its underlying mechanism. We demonstrated that the combination of NVP-BEZ235 and autophagy inhibitors has a synergic antitumor effect by promoting cell apoptosis and death both in vitro and vivo.
Since PI3K/Akt/mTOR signaling pathway plays a crucial role in tumorigenesis by strikingly promoting cell growth and proliferation (11,12), inhibition of this pathway is also postulated as a therapeutic option in LSCC (13). The mTOR protein serves as an integral part in this signaling complex, which is involved in two major complexes: mTOR complex1 (mTORC1) and mTOR complex2 (mTORC2) (14). Although discovered much later than mTORC1, mTORC2 is emerging as a promising candidate target in oncotherapy (15). Studies have proved that mTORC2 can directly regulate Akt phosphorylation via activating a critical regulatory site required for maximal Akt kinase activity, which enables Akt to become a crucial downstream protein of the mTORC2 signal pathway (16). Due to multiple resistance mechanisms, such as the feedback activation of the PI3K/Akt signaling network and the insensitivity of the mTORC1 complex, inhibition of the mTORC1 pathway has met with limited success in many studies (17,18), which is also verified in our experiment. Compared with Akt inhibitor LY294002 and mTORC1 inhibitor rapamycin, dual PI3K/mTOR inhibitor NVP-BEZ235 showed better anticancer effects in arresting the cell cycle, promoting cell apoptosis, and inhibiting cell invasion and metastasis by suppressing mTORC1 and mTORC2 at the same time. Changes in downstream proteins, such as p-Akt, p-S6RP, and p-p70S6K, also supported the anticancer effect of NVP-BEZ235 and its corresponding mechanism in two LSCC cell lines.
However, an inhibition of mTOR pathway, especially an inhibition of phosphorylation of mTOR, can also induced autophagy in head and neck squamous cell carcinomas (19). Through degradation of damaged proteins and organelles, autophagy can protect tumor cells from stressful conditions, thus mediating resistance to anticancer therapies such as radiation, chemotherapy, and targeted treatments (20,21). In our study, an increase of LC3II/I ratio was found in both LSCC cell lines treated with PI3K or mTOR inhibitors, among which the dual inhibitor NVP-BEZ235 is the most significant, supporting its induction of cell autophagy. Based on accumulating evidence that inhibition of autophagy can enhance the efficiency of antitumor drugs (22), we combined NVP-BEZ235 with autophagy inhibitors for further verification.
Surprisingly, the combination of NVP-BEZ235 with two autophagy inhibitors (3-MA, CQ) showed a synergetic antitumor effect in both cell lines. LSCC cells that inhibited autophagy in the early phase by silencing Atg7 also had increased cell apoptosis and death, further indicating that inhibition of autophagy can effectively improve the antitumor effect of PI3K/Akt inhibitors. We then combined NVP-BEZ235 with the less toxic autophagy inhibitor CQ to test their antitumor efficacy in vivo. The increased proportion of apoptotic cells in the tumor, along with the obviously decreased tumor size and tumor weight, were all consistent with those of our previous cell experiments.
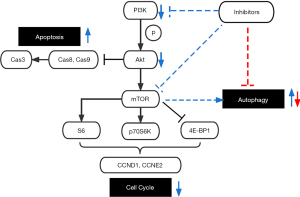
In conclusion, we have proved the remarkable antitumor effect of dual PI3K/Akt inhibitor NVP-BEZ235 compared to other PI3K or Akt inhibitors in LSCC cells, which may be achieved by inhibiting mTORC1 and mTORC2 simultaneously. Combined treatment with CQ, which could inhibit autophagy induced by NVP-BEZ235, further promoted tumor cell apoptosis and death both in vitro and vivo, indicating their potential synergistic antitumor effect in LSCC (Figure 7). Based on the current trend of combination therapy in cancer treatment (23), our experiments have solved the limitations of PI3K/Akt inhibitors to some extent, suggesting that combined application of autophagy inhibitors could be an effective way to overcome resistance of LSCC to PI3K/Akt inhibitors, while providing a promising therapy with the combined application of targeted drugs in LSCC.
Acknowledgments
We sincerely thank all the reviewers and editors for their supportive suggestions.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2325/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2325/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2325/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin 2017;67:31-50. [Crossref] [PubMed]
- National Comprehensive Cancer Network clinical practice guidelines in oncology, head and neck cancers, version 1.2021. Accessed: 11 March 2021. Available online: https://www.nccn.org/guidelines/patients
- Nwizu T, Adelstein D. Pharmacotherapy of head and neck cancer. Expert Opin Pharmacother 2015;16:2409-22. [Crossref] [PubMed]
- Zhou C, Liu C, Liu W, et al. SLFN11 inhibits hepatocellular carcinoma tumorigenesis and metastasis by targeting RPS4X via mTOR pathway. Theranostics 2020;10:4627-43. [Crossref] [PubMed]
- Martini M, De Santis MC, Braccini L, et al. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med 2014;46:372-83. [Crossref] [PubMed]
- Nuñez-Olvera SI, Gallardo-Rincón D, Puente-Rivera J, et al. Autophagy Machinery as a Promising Therapeutic Target in Endometrial Cancer. Front Oncol 2019;9:1326. [Crossref] [PubMed]
- Fan QW, Cheng C, Hackett C, et al. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal 2010;3:ra81. [Crossref] [PubMed]
- Akkoç Y, Berrak Ö, Arısan ED, et al. Inhibition of PI3K signaling triggered apoptotic potential of curcumin which is hindered by Bcl-2 through activation of autophagy in MCF-7 cells. Biomed Pharmacother 2015;71:161-71. [Crossref] [PubMed]
- Seitz C, Hugle M, Cristofanon S, et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 and chloroquine synergize to trigger apoptosis via mitochondrial-lysosomal cross-talk. Int J Cancer 2013;132:2682-93. [Crossref] [PubMed]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011;12:21-35. [Crossref] [PubMed]
- Xu F, Na L, Li Y, et al. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci 2020;10:54. [Crossref] [PubMed]
- Marquard FE, Jücker M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem Pharmacol 2020;172:113729. [Crossref] [PubMed]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149:274-93. [Crossref] [PubMed]
- Naruse T, Yanamoto S, Okuyama K, et al. Therapeutic implication of mTORC2 in oral squamous cell carcinoma. Oral Oncol 2017;65:23-32. [Crossref] [PubMed]
- Beauchamp EM, Platanias LC. The evolution of the TOR pathway and its role in cancer. Oncogene 2013;32:3923-32. [Crossref] [PubMed]
- Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene 2008;27:S43-51. [Crossref] [PubMed]
- Tamburini J, Chapuis N, Bardet V, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood 2008;111:379-82. [Crossref] [PubMed]
- Okuyama K, Suzuki K, Naruse T, et al. Prolonged cetuximab treatment promotes p27Kip1-mediated G1 arrest and autophagy in head and neck squamous cell carcinoma. Sci Rep 2021;11:5259. [Crossref] [PubMed]
- Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer 2017;17:528-42. [Crossref] [PubMed]
- He H, Song X, Yang Z, et al. Upregulation of KCNQ1OT1 promotes resistance to stereotactic body radiotherapy in lung adenocarcinoma by inducing ATG5/ATG12-mediated autophagy via miR-372-3p. Cell Death Dis 2020;11:883. [Crossref] [PubMed]
- Corallo D, Pastorino F, Pantile M, et al. Autophagic flux inhibition enhances cytotoxicity of the receptor tyrosine kinase inhibitor ponatinib. J Exp Clin Cancer Res 2020;39:195. [Crossref] [PubMed]
- Moghaddam SV, Abedi F, Alizadeh E, et al. Lysine-embedded cellulose-based nanosystem for efficient dual-delivery of chemotherapeutics in combination cancer therapy. Carbohydr Polym 2020;250:116861. [Crossref] [PubMed]


