
Harlequin syndrome induced by intraspinal analgesia in patients with advanced cancer: a case report
Introduction
Harlequin syndrome is a rare neurological disease that causes flushing on one side of the face, while the other side is pale and sweatless. Sometimes the arms and trunk may also be affected. Harlequin syndrome is believed to be due to the obstruction of the T2-T3 sympathetic vasodilator and sweat nerve (1). It is presumed that flushing and sweating are overcompensatory responses to the intact side of the sympathetic nerve to compensate for the loss of sweating and flushing on the side with sympathetic nerve dysfunction (2). We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2462/rc).
Case presentation
Herein, we report a case of Harlequin syndrome in a 30–40-year-old female who suffered from advanced colon cancer. The tumor metastasizes and compresses the retroperitoneal lymph nodes and nerves, causing persistent pain. We provided analgesia for the patient by placing a catheter in her epidural, burying the infusion port under the skin, and connecting with an analgesic pump (Figure 1).
The operation was successfully completed under lumbar anesthesia. During the operation, the patient received a 3 mL 0.2% bupivacaine + 1 mg morphine bolus to determine the block level and analgesic effect. After the operation, the patient returned to the recovery room and started treatment with a pulse-type analgesic pump. The mixture of bupivacaine and morphine was pumped in the same way. Two hours later, the nurse found that the patient had flushed on one side of the patient’s face, and the midline was clearly demarcated (Figure 2).
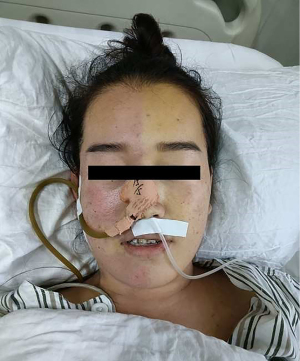
The accompanying symptoms were a temporary decrease in blood oxygen saturation and an increase in heart rate. Specifically, there was no ptosis or pupil reduction. And the patient did not complain of any numbness. After about 4 hours, the above series of symptoms resolved spontaneously. During this period, the patient felt no special discomfort and denied having similar symptoms in the past. The patient did not receive any corresponding treatment measures.
Except for colon cancer, the patient reported no history of any nervous system disease or history of traumatic accidents. And no definite abnormalities or space-occupying lesions were found from the patient’s preoperative contrast-enhanced head CT and trunk CT. Unfortunately, due to the patient’s indwelling metal contraceptive ring; and due to poor physical strength, the patient and her family refused to undergo further magnetic resonance imaging (MRI) examination/electromyography (EMG)/electroencephalography (EEG) after developing Harlequin syndrome.
This symptom reappeared 3 days later on the other side of her face, after we manually added the dose of the drug injected into the epidural cavity (Figure 3). The difference from the last time is that during the reappearance of Harlequin syndrome, the patient also experienced a transient drop in blood pressure. Again, it relieves itself like last time. Based on this, we have reason to speculate that the symptom was caused by spinal canal analgesia.
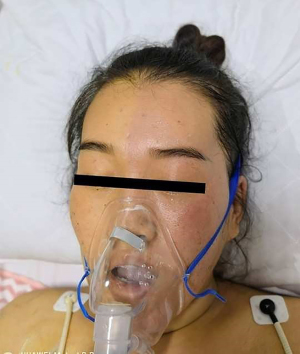
We subsequently tried to reduce the pumping speed of analgesic drugs in the spinal canal and shorten the interval between the application of analgesic drugs to the patient. After we adjusted the delivery mode of the analgesic pump, the patient did not have the above symptoms in the subsequent follow-up. The patient continued her palliative care in our hospital for the next 45 days, after which the patient passed away. We suspect that the short-term large amount of anesthesia and analgesic drugs entered the spinal canal and extended to the nerve roots and infiltrated the sympathetic nerves of the higher segment, which caused the patient to have a series of comprehensive manifestations including Harlequin syndrome, decreased respiratory function and increased heart rate.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The clinically invasive procedures involved in this case report were all signed by the patient. Written informed consent was obtained from the patient’s family for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Harlequin syndrome is usually benign and is believed to be caused by T2-T3 vasomotor and sympathetic disruption of sweat fibers. It may involve parasympathetic neurons in the posterior ganglia and ciliary ganglia (3).
From an anatomical point of view, the sympathetic nerve conduction pathway descends along the spinal cord to the preganglionic neuron located in the gray matter lateral horn of the spinal cord from C8 to T3, and enters the paravertebral stellate ganglia to innervate the cheek and forehead. When the lesion is close to the stellate ganglion, it will affect the sweat fibers and vasomotor fibers that innervate the arms, neck, upper limbs, and upper trunk (4). When the lesion is at the distal end of the stellate ganglion, only the face is affected (5). Lesions localized between the stellate ganglion and the superior cervical ganglion may present with both Harlequin’s sign and Horner’s syndrome without involvement of the upper extremities and trunk (6).
In this case, the patient underwent a low epidural anesthesia. The tube was placed caudally. The level of anesthesia was measured below T8. The sympathetic nerve response was often almost 2–6 spinal segments higher than the sensory level. It was judged that the sympathetic block level might have reached T2-T3.
The iatrogenic type of Harlequin syndrome, like our case, can be seen after internal jugular vein catheterization (7), after paravertebral thoracic anesthesia (8), after surgical removal of a neck mass (9), or after chest surgery (10). However, no cases of flushing on different sides of the face in the same patient have been found. We believe that the occurrence of this phenomenon may be related to the drift of the spinal canal. Or pulse injection of analgesic and anesthetic drugs made the drugs to float in the soft tissues, resulting in differences in drug concentration at different nerve roots. This case proves that Harlequin syndrome caused by repeated drug stimulation could be reversible and variable in position.
Drug stimulation may also cause Horner syndrome-related manifestations. A research analysis specifically for the transient Horner’s sign after intraspinal anesthesia in obstetrics found that the incidence rate reached 0.4% to 2.5%. To study the cause of the occurrence, MRI examination of the neck and spinal canal was performed, and it was found that some patients had an epidural catheter inserted into the subdural space; 1 patient was found to have a carotid artery dissection. The application of high-density local anesthetics and other related factors have also been considered as the cause of transient Horner’s sign induced by intraspinal anesthesia (11). Thomas et al. (12) reported 2 cases of Harlequin syndrome after thoracic epidural analgesia. He believes that Harlequin syndrome is the result of sympathetic nerve conduction blocking the blood vessels of the lateral and upper limbs.
Most Harlequin syndrome cases are idiopathic, but they may also be iatrogenic or caused by space-occupying lesions or central nervous system infarction. From changes in sympathetic nerve excitability to sympathetic preganglionic fiber damage, unknown trauma to the superior cervical ganglion or the post-sympathetic ganglion fiber external carotid plexus are all causes of abnormal autonomic nerve function. Clinicians should be aware of this condition in case there is an underlying disease (13).
To diagnose properly and provide multidisciplinary assistance, despite the rarity of Harlequin syndrome, predisposing causes of Harlequin syndrome should be excluded before the disease is diagnosed as the primary cause.
Limited to the conditions at the time, the patient in this case did not undergo MRI examination of the head and spine. Instead, the patient’s CT scan of the head and spine did not show clear lesions. However, it is impossible to rule out the possibility of abnormalities in the nervous system and accompanying blood vessels caused by cervical spine disease. This is an unsatisfactory part of this case.
In conclusion, we found that Harlequin syndrome could be induced by intraspinal analgesia in patients with advanced cancer. And if the syndrome is related to anesthesia, reducing or stopping the infusion of local anesthesia should resolve the symptoms. We should keep in mind that there are many Harlequin syndrome cases that can be ignored due to atypical symptoms. If necessary, patients must be required to undergo imaging examinations of the head and spinal cord to rule out the possibility of hidden diseases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2462/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2462/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2462/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The clinically invasive procedures involved in this case report were all signed by the patient. Written informed consent was obtained from the patient’s family for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psychiatry 1988;51:635-42. [Crossref] [PubMed]
- Lefevre A, Schnepper G. Development of Harlequin Syndrome following placement of thoracic epidural anesthesia in a pediatric patient undergoing Nuss procedure. Clin Case Rep 2017;5:1523-5. [Crossref] [PubMed]
- Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 2003;78:603-12. [Crossref] [PubMed]
- Wilks DJ, Urso-Baiarda F, Thornton D, et al. Re: Coexisting harlequin and Horner syndromes after paediatric neck dissection: a case report and a review of the literature. J Plast Reconstr Aesthet Surg 2009;62:269-70. [Crossref] [PubMed]
- Tang X, Liu J, Yin H. Epidural Anesthesia-induced Harlequin Syndrome: a Case Report. Modern Hospital 2016;16:1293-4.1298.
- Fringeli Y, Humm AM, Ansorge A, et al. Harlequin sign concomitant with Horner syndrome after anterior cervical discectomy: a case of intrusion into the cervical sympathetic system. J Neurosurg Spine 2017;26:684-7. [Crossref] [PubMed]
- Coleman PJ, Goddard JM. Harlequin syndrome following internal jugular vein catheterization in an adult under general anesthetic. Anesthesiology 2002;97:1041. [Crossref] [PubMed]
- Nagasaka Y, Wasner G, Sharma B, et al. Harlequin Syndrome After Thoracic Paravertebral Block. A A Case Rep 2016;6:48-51. [Crossref] [PubMed]
- Lee DH, Seong JY, Yoon TM, et al. Harlequin syndrome and Horner syndrome after neck schwannoma excision in a pediatric patient: A case report. Medicine (Baltimore) 2017;96:e8548. [Crossref] [PubMed]
- Sribnick EA, Boulis NM. Treatment of Harlequin syndrome by costotransversectomy and sympathectomy: case report. Neurosurgery 2011;69:E257-9. [Crossref] [PubMed]
- Barbara R, Tome R, Barua A, et al. Transient Horner syndrome following epidural anesthesia for labor: case report and review of the literature. Obstet Gynecol Surv 2011;66:114-9. [Crossref] [PubMed]
- Thomas JJ, Polaner D. Harlequin Syndrome Associated with Thoracic Epidural Analgesia. Anesthesiology 2015;123:1187. [Crossref] [PubMed]
- Willaert WI, Scheltinga MR, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg 2009;109:214-20. [PubMed]



