Comparison of efficacy and safety of bevacizumab biosimilar and original bevacizumab in non-squamous non-small cell lung cancer: a systematic review and meta-analysis
Introduction
Lung cancer is currently the most common cause of cancer-related death worldwide. Non-small cell lung cancer (NSCLC) is the most dominant histological subtype of all primary lung cancer, accounting for 85% of all cases (1-3). The 5-year survival rate for lung cancer varies from 4% to 17% (4), and the unsatisfactory prognosis for NSCLC is strongly associated with frequent recurrence, lymph node metastasis, and distant metastasis. Therefore, to explore a set of effective and cheap treatment has always been the goal of researchers and clinicians (5).
Angiogenesis is an important feature in tumor pathogenesis and targeting angiogenesis has always been a hot topic in the research and development of anti-tumor drugs. Vascular endothelial growth factors (VEGFs), especially VEGF-A, is one of the key factors promoting tumor angiogenesis. Previous studies have shown that VEGF contributed to abnormal hematopoiesis, inhibited activated T cells, and promoted immunosuppressive cells by activating vascular endothelial growth factor receptor (VEGFR) (6-9). Therefore, targeting VEGF/VEGFR is considered to be an effective anti-tumor method.
Bevacizumab is a humanized monoclonal antibody synthesized by recombinant DNA technology, which is the first anti-tumor angiogenesis drug targeting VEGF in the world. By binding to VEGF, bevacizumab can inhibit the combination of VEGF and VEGFR, block VEGF/VEFGR pathway, and inhibit the growth of tumor cells (10). Bevacizumab, an important anti-angiogenesis drug, has been approved for use in a variety of solid tumors, including NSCLC, metastatic colon cancer, recurrent glioblastoma, metastatic renal cell carcinoma, and ovarian cancer (11-15). Multiple RCTs have confirmed that bevacizumab combined with chemotherapy was relayed with better OS and PFS compared to using chemotherapy alone (16-18). Despite these benefits, patients’ access to bevacizumab may be limited due to high cost of the drug and health insurance restrictions (19).
Biosimilar, a biological agent similar to the approved original drug, has strong similarity to the approved original drug in terms of quality, safety and efficacy (20). In a sense, biosimilars are becoming an alternative to expensive original drugs because of their low cost and similar efficacy. Biosimilars have important economic and social benefits such as reducing medical expenses, increasing access to drugs and improving medical service level (21,22). To date, at least 7 types of biosimilar bevacizumab have been approved in different countries, of which two (Mvasi™ and Zirabev™) have been approved by European Medicines Agency (EMA) and Food and Drug Administration (FDA). These drugs have been approved for the following indications: metastatic colorectal cancer; advanced, recurrent, or metastatic NSCLC; glioblastoma recurrence; metastatic renal cell carcinoma; persistent, recurrent or metastatic cervical cancer and ophthalmology (23,24).
Although the approval of biosimilars is accelerating, many clinicians are still unfamiliar with the concept of biosimilars, which hinders the development and application of biosimilars. In addition, the lack of data on the efficacy and safety of biosimilars compared with the original drugs further exacerbates this situation. Therefore, objective and systematic evidence is required to promote the application of biosimilars (25).
To our knowledge, there is no meta-analysis on NSCLC comparing the efficacy and safety of biosimilar bevacizumab with the original drug. Therefore, we aimed to conduct a systematic literature review and meta-analysis of published RCTs and compare the efficacy and safety of biosimilar bevacizumab with the original drug. We present the following article in accordance with the PRISMA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-71/rc) (26).
Methods
A protocol of this review is under assessment of the PROSPERO registration No. CRD42021276991 (https://www.crd.york.ac.uk/prospero/). Title registered for the protocol: Comparison of efficacy and safety of bevacizumab biosimilar and original bevacizumab in non-squamous non-small cell lung cancer: a systematic review and meta-analysis.
Data source and literature search
The Web of Science, PubMed, Cochrane Library, EMBASE, and ClinicalTrials.gov electronic databases were searched until 15 October 2021 to identify eligible articles using a comprehensive search strategy with relevant keywords and medical subject headings (MeSH). Additional search was conducted on ClinicalTrials.gov to reduce potential publication bias and identify ongoing trials and extended studies. Titles and abstracts were distinguished by using the following search terms to sort out relevant texts: “biosimilar*”, “Follow-on Biologics”, “Biologics, Subsequent Entry”, “bevacizumab”, “Avastin”, “Mvasi”, “Non-Small-Cell Lung Carcinoma*”, and “Non-Small Cell Lung Cancer”.
Study eligibility and selection
All RCTs comparing the efficacy and safety of biosimilar bevacizumab and the original drug in NSCLC were selected and evaluated for inclusion in the study.
The inclusion criteria were as follows:
- Randomized, controlled, double-blind trials; biosimilar bevacizumab used as the experimental group and original bevacizumab as controlled group;
- Designed to treat patients with NSCLC;
- Included at least 30 participants;
- Detailed results of treatment-related response rates, free-of-attack rates, discontinuation and adverse event (AE) reports.
The exclusion criteria were as follows:
- Not RCTs;
- For treatment of other diseases rather than NSCLC;
- Did not report detailed data to evaluate the efficacy and safety;
- Conference abstracts, editorials, letters, and reviews;
- Previous studies using the same data as a more recent study.
Two reviewers independently searched and screened eligible articles by title, abstract, and/or full text. Any inconsistencies or uncertainties were resolved by consensus.
Data extraction and quality assessment
Two reviewers (Xiao and Zhang) independently extracted the following data from the abstractions, forms, tables, primary texts, and supplementary appendixes: first author, year of publication, phase of the trial, areas of trial conducted, mean age and male/female ratio, therapeutic regimens, main outcomes, details of withdrawals and serious AEs related to treatments, the most common AEs, analysis principle, and ClinicalTrials.gov identifier. Cochrane Collaboration’s tool were used to assess the quality of included trials (27). Bias risk of each study was classified as high, low, or unclear based on random sequence generation, assignment hiding, outcome participant and person blindness, outcome assessment blindness, outcome data incomplete, selective reporting, etc. The literature search and subsequent article evaluation were carried out independently by two reviewers (Xiao and Zhang), and any disagreement was determined by a third reviewer (Sun).
Statistical analysis
The clinical response was evaluated in terms of treatment effect and AEs. Treatment efficacy was evaluated by using objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and AEs including serious AEs, neutropenia, leukopenia, alopecia, anemia, hypertension, nausea, proteinuria. Statistical heterogeneity was evaluated using Cochran’s Q test, and significant heterogeneity was defined as P<0.10. The heterogeneity across studies was considered to be moderate or high if the I2 statistic was greater than 50%. A pooled RR was calculated in a random effects model using DerSimonian and Laird method if the heterogeneity was significant (P<0.10), otherwise the fixed effects model was chosen, which was based on the Mantel-Haenszel method. We synthesized the summary estimates of risk ratio (RR) and corresponding 95% confidence interval (95% CI). The reported HRs were transferred to RRs, which were used for final meta-analyses after transformation (28). A subgroup analysis was performed based on whether the Chinese experiment or the multicenter experiment. In order to test the robustness of overall effect sizes, one study was omitted each time. Sensitivity analyses were performed to evaluate the influence of each individual study on the overall effect sizes. One single study was considered as having influence on the overall effect size, if the point estimate of the “omitted” analysis lay outside of the 95% CI of the “combined” analysis. Publication bias was evaluated by Egger’s test and significant publication bias was defined as P<0.05. All analyses were performed using STATA 12.0 (StataCorp, Texas, USA) and RevMan 5.3.0.
Results
Literature search
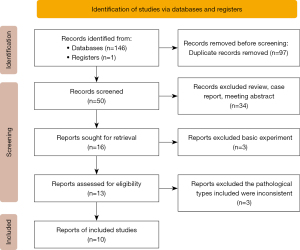
According to the pre-set search strategy, a total of 147 results were found, and 97 duplicate references were excluded. After reviewing the full text of the remaining 50 literatures, 10 randomized controlled trials (RCTs) with 6,416 participants were finally included (29-38). The detailed literature selection process was shown in Figure 1.
Characteristics of included studies
Table 1 summarized the basic characteristics of 10 RCTS. All of the studies were phase III clinical trials completed between 2019 and 2021. Three of the studies were conducted in China, and the remaining 7 were conducted in multiple countries. A total of 6,416 patients were enrolled, with 3,220 patients receiving bevacizumab biosimilar in combination with paclitaxel and carboplatin in the experimental group and 3,188 patients in the control group receiving bevacizumab monoantigen in combination with paclitaxel and carboplatin. The mean age of the experimental group was 60.09, and that of the control group was 60.00. The proportion of male in the experimental group was 63.89%, and that in the control group was 62.64%. Biosimilar bevacizumab in all the RCTs were from different brands. The treatment lasted 4–6 cycles in both the experimental group and the control group. In the same RCTs study, patients in the experimental group and the control group received the same chemotherapy regimen and dosage. The patient characteristics were shown in Table 1.
Table 1
| Study | Phase | Year | Brand name | Country | N, Exp/Con | Gener (male) Exp/Con | Age years (mean ± SD) Exp/Con | Combined with chemotherapy or not | Duration (months) | Outcomes measure | Identifier |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chu T1 | III | 2021 | QL1101 | China | 269/266 | 158 (58.7%)/ 160 (60.2%) |
58.5±8.48/ 57.8±7.07 |
Y | 22.4 | ORR, PFS, OS, DCR, AEs | NCT03169335 |
| Reck M2 | III | 2020 | SB8 | Multicenter (13 countries) |
397/384 | 252 (66.5%)/ 256 (66.7%) |
60.2±8.95/ 60.0±9.18 |
Y | 15.2 | ORR, PFS, OS, DOR, AEs | NCT02754882 |
| Syrigos K3 | III | 2021 | FKB238 | Multicenter (NA) |
364/367 | 245 (67.3%)/ 238 (64.9%) |
60.8±8.79/ 61.1±9.42 |
Y | 4.75 | ORR, PFS, OS, AEs | NCT02810457 |
| Reinmuth N4 | III | 2019 | PF-06439535 | Multicenter (27 countries) |
358/361 | 237 (66.2%)/ 230 (63.7%) |
61.7±10.61/ 60.8±8.89 |
Y | 6.25 | ORR, PFS, OS, DOR, AEs | NCT02364999 |
| Thatcher N5 | III | 2019 | ABP 215 | Multicenter (17 countries) |
328/314 | 196 (59.8%)/ 188 (59.9%) |
61.6±9.09/ 61.6±8.88 |
Y | 4.75 | ORR, PFS, OS, DOR, AEs | NCT01966003 |
| Shi Y6 | III | 2021 | LY01008 | China | 293/296 | 177 (60.4%)/ 175 (59.1%) |
58±NA/ 59±NA |
Y | 28.4 | ORR, PFS, OS, DOR, AEs | NCT03533127 |
| Trukhin D7 | III | 2021 | MB02 | Multicenter (16 countries) |
315/312 | 193 (61.3%)/ 190 (60.9%) |
60.5±9.02/ 60.5±9.38 |
Y | 4.5 | ORR, PFS, OS, immunogenicity, AEs | NCT03296163 |
| Yang Y8 | III | 2019 | IBI305 | China | 224/226 | 137 (62.3%)/ 142 (64.3%) |
57.6±8.69/ 57.2±8.28 |
Y | 18 | ORR, PFS, OS, DOR, AEs | NCT02954172 |
| NCT046335649 | III | 2021 | MYL-1402O | Multicenter (NA) |
337/334 | 213 (63.2%)/ 211 (63.2%) |
59.3±9.60/ 59.2±9.73 |
Y | 4.5 | ORR | NCT04633564 |
| Kim ES10 | III | 2021 | BI 695502 | Multicenter (NA) |
335/328 | 214 (63.9%)/ 203 (61.9%) |
61.2±9.89/ 61.3±9.22 |
Y | 4.5 | ORR, PFS, OS, DOR, AEs | NCT02272413 |
1, paclitaxel (175 mg/m2 Q21d IV) plus carboplatin (AUC 6 Q21 IV) for 4–6 cycles; 2, paclitaxel (200 mg/m2 Q21d IV) plus carboplatin (AUC 6 Q21 IV) for 4–6 cycles; 3, paclitaxel (200 mg/m2 Q21d IV) plus carboplatin (AUC 6 Q21 IV) for 4–6 cycles; 4, paclitaxel (200 mg/m2 Q21d IV) plus carboplatin (AUC 6 Q21 IV) for 4–6 cycles; 5, paclitaxel plus carboplatin for 4–6 cycles; 6, paclitaxel (175 mg/m2 Q21d IV) plus carboplatin (AUC 6 Q21 IV) for 4–6 cycles; 7, paclitaxel (200 mg/m2 Q21d IV) plus carboplatin (AUC 6 Q21 IV) for 6 cycles; 8, paclitaxel (175 mg/m2 Q21d IV) plus carboplatin (AUC 6 Q21 IV) for 6 cycles; 9, paclitaxel (175 or 200 mg/m2 Q21d IV) plus carboplatin (AUC 6 Q21 IV) for 6 cycles; 10, paclitaxel (200 mg/m2 Q21d IV) plus carboplatin (AUC 6 Q21 IV) for 6 cycles. exp, experimental group; con, control group; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; DCR, disease control rate; DOR, duration of response; AE, adverse event; NA, not available; AUC, area under the receiver operating characteristic curve.
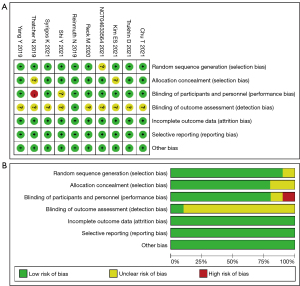
Quality assessment for included studies
Our bias risk analysis using the Cochrane Collaboration tool showed bias in all the included studies. “Randomization”, “randomization” or “randomization” were mentioned in all the nine studies, and the assignment of concealment schemes and blind evaluation of study outcomes were clearly specified in the report. A total of eight studies applied blinding to both investigators and subjects. All the included studies reported detailed outcome data. The risk of biased assessment was shown in Figure 2.
Outcomes measures
Efficacy
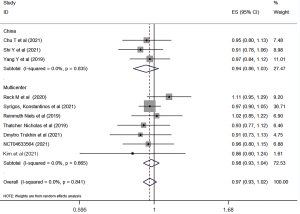
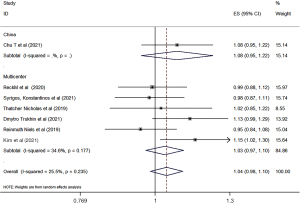
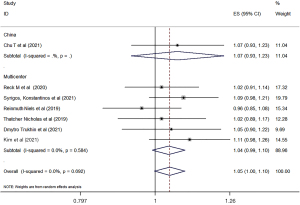
The clinical response rates of biosimilars bevacizumab and the original bevacizumab were analyzed according to the China or world multicenter research. A total of ten studies were included to compare the clinical outcomes of biosimilars with those of the original bevacizumab. All of the studies reported the ORR of biosimilar bevacizumab and the original bevacizumab, and 7 studies (29-34,38) reported the PFS and OS. Overall, there was no significant difference in ORR, PFS, or OS between biosimilar bevacizumab and original bevacizumab, whether the Chinese experiment or the multicenter experiment (Figures 3-5). All analyses showed low heterogeneity between studies (ORR: RR 0.97, 95% CI: 0.93–1.02, P=0.841, I2=0; PFS: RR 1.04, 95% CI: 0.98–1.10, P=0.235, I2=0; OS: RR 1.05, 95% CI: 1.00–1.10, P=0.692, I2=0). Sensitivity analyses were performed to evaluate the stability of the overall effect size, and no outlier was detected (Figures S1-S3).
Safety
The incidence of AEs in biosimilar bevacizumab and original bevacizumab were analyzed using all the included studies. Overall, there was no significant difference in the incidence of adverse reactions between biosimilar bevacizumab and original bevacizumab. The pooled incidence rate of serious AEs was 0.25 (0.21–0.30) for biosimilar bevacizumab and 0.25 (0.21–0.30) for the original bevacizumab, indicating that there was no difference in the incidence of severe AEs between the two treatments (Figure S4). The heterogeneity between studies was low. For the remaining AEs (including neutropenia, leukopenia, alopecia, anemia, hypertension, nausea, proteinuria), there was no significant difference between the two groups (Figures S5-S11).
Discussion
Lung cancer is a leading cause of cancer-related death worldwide, and NSCLC accounts for about 85% of lung cancer cases (39). Antiangiogenic drugs have been proved to be effective in treating malignancy, and bevacizumab is one of the main representatives of antiangiogenic drugs. Since FDA approved bevacizumab for the treatment of non-squamous NSCLC in October 2006, bevacizumab has attracted extensive attention and demonstrated encouraging therapeutic effects (40-42). However, due to the production process and patent protection, the high price of bevacizumab has hindered the application of bevacizumab and the treatment of non-squamous NSCLC to a certain extent (41). However, the emergence of biocontrol drugs has brought hope to solve this dilemma. Biosimilars are gradually gaining popularity due to their similar efficacy with the original drugs and low price, and they have also been approved by FDA (43). Both American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) have adopted guidelines that encourage cost-effective treatment of cancer (6). In recent years, biosimilar bevacizumab has been developed gradually and achieved good results. Therefore, this study aimed to evaluate the efficacy and safety of biosimilar bevacizumab versus the original drug in patients with non-squamous NSCLC by collating available data from head-to-head RCTs.
In our study, we compared biosimilar bevacizumab and original bevacizumab in combination with paclitaxel plus carboplatin. Ten RCTs were included in our research involving 6,416 NSCLC patients, and the analysis showed no significant difference in efficacy and safety between biosimilar bevacizumab and the original bevacizumab. The quality of the evidence was rated as medium to high. As shown by subgroup analysis, there was no difference in the effects of biosimilars in either Chinese or multicenter studies. The results were proved to be reliable by sensitivity analysis.
To the best of our knowledge, this is the first systematic review comparing the efficacy and safety of biosimilar bevacizumab with the original bevacizumab in the treatment of NSCLC. In a previous meta-analysis conducted by Yang et al. (44), a comparison was made between the application of biosimilar bevacizumab and its original drug in multiple cancer types, among which there were only 3 RCTs related to NSCLC. One of the studies was published as a conference paper (45), and the other two articles were included in our study (29,31). In their research, the results showed no significant difference between the biosimilar bevacizumab and its original drugs in efficacy and safety (ORR: RR 0.96, 0.81–1.14; AEs rate: RR 1.01, 0.98–1.03). Our results were consistent with previous research, which proved the effectiveness of our research.
Typically, the primary endpoint of anticancer activity in cancer specific therapies includes PFS or OS, but this might not be sufficiently sensitive to biosimilars compared to the original drug. Therefore, EMA recommended the use of clinical endpoint as the primary measure of efficacy (i.e., ORR is usually used) (46). In our study, ORR was reported in all RCTs studies, and the follow-up time of the study ranged from 4.5 to 28.4 months. We conducted subgroup analysis based on the whether the Chinese experiment or the multicenter experiment, and the analysis results showed that there was no significantly statistical difference between biosimilars compared with the original drugs in either Chinese or multicenter studies (China RR: 0.94, 0.86–1.03; Multicenter RR: 0.98, 0.93–1.04; total RR: 0.97, 0.93–1.02). Similar results were found in PFS and OS. In terms of PFS and OS data reported by 7 studies, there was no significantly statistical difference between biosimilar bevacizumab and its original drugs.
Previous studies have shown that bevacizumab combined with chemotherapy could cause typical common adverse reactions such as alopecia, peripheral neuropathy, rash, proteinuria, nausea, fatigue, myalgia, bleeding, and hypertension (47). Common and typical AEs reported in all RCTS were included in our study (including neutropenia, leukopenia, alopecia, anemia, hypertension, nausea, proteinuria), and the analysis showed no significant difference in the incidence of adverse reactions between biosimilar bevacizumab and original drugs. The pooled incidence rate of serious AEs was 0.24 (0.19–0.29) for biosimilar bevacizumab and 0.23 (0.18–0.29) for the original bevacizumab. There was a crossover in the incidence of pooling between biosimilar bevacizumab and original drugs, indicating no significant difference between the two groups. Similar results were obtained in other AEs. The incidence of alopecia was the highest in both groups (alopecia: biosimilars: 0.46, 95% CI: 0.44–0.48; original drug: 0.48, 95% CI: 0.45–0.50).
Unfortunately, there are several limitations in our research. First, some key clinical data (such as OS and PFS) have not been reported in all the included RCTs. Second, the number of clinical trials included in our study was small, which might lead to reduced applicability in different populations and routine clinical settings. Third, all the included studies used biosimilar bevacizumab of different brands, which made it impossible to conduct subgroup analysis based on biosimilars brand. We still need to pay attention to the efficacy and safety of the products from different brands in the future. Finally, the average age of the study population included in the experimental group and the control group was not more than 65 years old. Further studies on the effectiveness and safety of biosimilars in the elderly are needed in the future.
Conclusions
Biosimilars have attracted more and more attention due to their low price and similar effects with the original drugs. It is extremely important to evaluate the efficacy and safety of biosimilars and the original drugs. We performed a meta-analysis using the included RCTS to evaluate the equivalence between biosimilar bevacizumab and the original drug. The analysis showed no significant difference in ORR, PFS and OS between biosimilar bevacizumab and the original drug. In terms of safety, pooled incidence rate of serious AEs between biosimilars and the original drug was similar, and there was no significant difference between biosimilar bevacizumab and the original drug. Same results were found in some common adverse reactions (including neutropenia, leukopenia, alopecia, anemia, hypertension, nausea, proteinuria).
In summary, biosimilar bevacizumab are similar to the original drugs in terms of efficacy and safety, and can be considered as a substitute for bevacizumab in the treatment of NSCLC.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-71/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-71/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang Z, Tang H, Chen P, et al. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct Target Ther 2019;4:41. [Crossref] [PubMed]
- Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 2017;17:637-58. [Crossref] [PubMed]
- Thomas A, Liu SV, Subramaniam DS, et al. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat Rev Clin Oncol 2015;12:511-26. [Crossref] [PubMed]
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Tan WL, Chua KLM, Lin CC, et al. Asian Thoracic Oncology Research Group Expert Consensus Statement on Optimal Management of Stage III NSCLC. J Thorac Oncol 2020;15:324-43. [Crossref] [PubMed]
- Balaban S, Shearer RF, Lee LS, et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab 2017;5:1. [Crossref] [PubMed]
- Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol 2018;9:978. [Crossref] [PubMed]
- Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol 2018;52:117-24. [Crossref] [PubMed]
- Rahma OE, Hodi FS. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin Cancer Res 2019;25:5449-57. [Crossref] [PubMed]
- Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev 2020;86:102017. [Crossref] [PubMed]
- Des Guetz G, Uzzan B, Nicolas P, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 2006;94:1823-32. [Crossref] [PubMed]
- Seto T, Higashiyama M, Funai H, et al. Prognostic value of expression of vascular endothelial growth factor and its flt-1 and KDR receptors in stage I non-small-cell lung cancer. Lung Cancer 2006;53:91-6. [Crossref] [PubMed]
- Flynn JR, Wang L, Gillespie DL, et al. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer 2008;113:1032-42. [Crossref] [PubMed]
- Paley PJ, Staskus KA, Gebhard K, et al. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer 1997;80:98-106. [Crossref] [PubMed]
- Gao X, McDermott DF. Combinations of Bevacizumab With Immune Checkpoint Inhibitors in Renal Cell Carcinoma. Cancer J 2018;24:171-9. [Crossref] [PubMed]
- Cella D, Wang M, Wagner L, et al. Survival-adjusted health-related quality of life (HRQL) among patients with metastatic breast cancer receiving paclitaxel plus bevacizumab versus paclitaxel alone: results from Eastern Cooperative Oncology Group Study 2100 (E2100). Breast Cancer Res Treat 2011;130:855-61. [Crossref] [PubMed]
- Qin S, Ren Z, Feng YH, et al. Atezolizumab plus Bevacizumab versus Sorafenib in the Chinese Subpopulation with Unresectable Hepatocellular Carcinoma: Phase 3 Randomized, Open-Label IMbrave150 Study. Liver Cancer 2021;10:296-308. [Crossref] [PubMed]
- Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol 2015;16:928-36. [Crossref] [PubMed]
- Monk BJ, Lammers PE, Cartwright T, et al. Barriers to the Access of Bevacizumab in Patients with Solid Tumors and the Potential Impact of Biosimilars: A Physician Survey. Pharmaceuticals (Basel) 2017;10:19. [Crossref] [PubMed]
- Organization WH. Guidelines on evaluation of similar biotherapeutic products (SBPs) 2009 Available online: https://www.who.int/publications/m/item/sbp-trs-977-Annex-2
- Gulácsi L, Brodszky V, Baji P, et al. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev Clin Immunol 2015;11:S43-52. [Crossref] [PubMed]
- Mehr SR, Brook RA. Biosimilars in the USA: Will New Efforts to Spur Approvals and Access Spur Uptake and Cost Savings? Pharmaceut Med 2019;33:1-8. [Crossref] [PubMed]
- Biosimilars of trastuzumab. Generics and biosimilars initiative (GaBi). Mol, Belgium 2020;2019:18.
- Casak SJ, Lemery SJ, Chung J, et al. FDA's Approval of the First Biosimilar to Bevacizumab. Clin Cancer Res 2018;24:4365-70. [Crossref] [PubMed]
- Leonard E, Wascovich M, Oskouei S, et al. Factors Affecting Health Care Provider Knowledge and Acceptance of Biosimilar Medicines: A Systematic Review. J Manag Care Spec Pharm 2019;25:102-12. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- VanderWeele TJ. On a Square-Root Transformation of the Odds Ratio for a Common Outcome. Epidemiology 2017;28:e58-60. [Crossref] [PubMed]
- Reinmuth N, Bryl M, Bondarenko I, et al. PF-06439535 (a Bevacizumab Biosimilar) Compared with Reference Bevacizumab (Avastin®), Both Plus Paclitaxel and Carboplatin, as First-Line Treatment for Advanced Non-Squamous Non-Small-Cell Lung Cancer: A Randomized, Double-Blind Study. BioDrugs 2019;33:555-70. [Crossref] [PubMed]
- Syrigos K, Abert I, Andric Z, et al. Efficacy and Safety of Bevacizumab Biosimilar FKB238 Versus Originator Bevacizumab: Results from AVANA, a Phase III Trial in Patients with Non-Squamous Non-Small-Cell Lung Cancer (non-sq-NSCLC). BioDrugs 2021;35:417-28. [Crossref] [PubMed]
- Thatcher N, Goldschmidt JH, Thomas M, et al. Efficacy and Safety of the Biosimilar ABP 215 Compared with Bevacizumab in Patients with Advanced Nonsquamous Non-small Cell Lung Cancer (MAPLE): A Randomized, Double-blind, Phase III Study. Clin Cancer Res 2019;25:2088-95. Erratum in: Clin Cancer Res 2019;25:3193. [Crossref] [PubMed]
- Trukhin D, Poddubskaya E, Andric Z, et al. Efficacy, Safety and Immunogenicity of MB02 (Bevacizumab Biosimilar) versus Reference Bevacizumab in Advanced Non-Small Cell Lung Cancer: A Randomized, Double-Blind, Phase III Study (STELLA). BioDrugs 2021;35:429-44. [Crossref] [PubMed]
- Chu T, Lu J, Bi M, et al. Equivalent efficacy study of QL1101 and bevacizumab on untreated advanced non-squamous non-small cell lung cancer patients: a phase 3 randomized, double-blind clinical trial. Cancer Biol Med 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Reck M, Luft A, Bondarenko I, et al. A phase III, randomized, double-blind, multicenter study to compare the efficacy, safety, pharmacokinetics, and immunogenicity between SB8 (proposed bevacizumab biosimilar) and reference bevacizumab in patients with metastatic or recurrent nonsquamous non-small cell lung cancer. Lung Cancer 2020;146:12-8. [Crossref] [PubMed]
- Yang Y, Wu B, Huang L, et al. Biosimilar candidate IBI305 plus paclitaxel/carboplatin for the treatment of non-squamous non-small cell lung cancer. Transl Lung Cancer Res 2019;8:989-99. [Crossref] [PubMed]
- Shi Y, Lei K, Jia Y, et al. Bevacizumab biosimilar LY01008 compared with bevacizumab (Avastin) as first-line treatment for Chinese patients with unresectable, metastatic, or recurrent non-squamous non-small-cell lung cancer: A multicenter, randomized, double-blinded, phase III trial. Cancer Commun (Lond) 2021;41:889-903. [Crossref] [PubMed]
- Nct. MYL-1402O Compared With Avastin®, in Patients With Stage IV nsNSCLC. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04633564
- Kim ES, Balser S, Rohr KB, et al. Phase 3 Trial of BI 695502 Plus Chemotherapy Versus Bevacizumab Reference Product Plus Chemotherapy in Patients With Advanced Nonsquamous NSCLC. JTO Clin Res Rep 2021;3:100248. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Huang HY, Wu DW, Ma F, et al. Availability of anticancer biosimilars in 40 countries. Lancet Oncol 2020;21:197-201. [Crossref] [PubMed]
- Lyman GH, Zon R, Harvey RD. Rationale, Opportunities, and Reality of Biosimilar Medications. N Engl J Med 2018;379:694-5. [Crossref] [PubMed]
- Santos SB, Sousa Lobo JM, Silva AC. Biosimilar medicines used for cancer therapy in Europe: a review. Drug Discov Today 2019;24:293-9. [Crossref] [PubMed]
- FDA Approves First Biosimilar to Treat Cancer. Cancer Discov 2017;7:1206.
- Yang J, Yu S, Yang Z, et al. Efficacy and Safety of Anti-cancer Biosimilars Compared to Reference Biologics in Oncology: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BioDrugs 2019;33:357-71. [Crossref] [PubMed]
- Filon O, Orlov S, Burdaeva O, et al. Efficacy and safety of BCD-021, bevacizumab biosimilar candidate, compared to Avastin: Results of international multicenter randomized double blind phase III study in patients with advanced non-squamous NSCLC. J Clin Oncol 2015;33:8057. [Crossref]
- EMA. Guideline on similar biological medicinal products containing monoclonal antibodies – non-clinical and clinical issues. European Medicines Agency; 1 Nov 2016.
- Zhao X, Wang M, Zhang L, et al. Evaluation of efficacy and safety of bevacizumab combined with chemotherapy for Chinese patients with advanced non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2012;15:6-10. [PubMed]