
A network-based pharmacological study on the mechanism of action of muscone in breast cancer
Introduction
Breast cancer occurs when breast epithelial cells proliferate uncontrollably under the action of multiple oncogenic factors. According to the latest global cancer burden projections for 2020, Breast cancer accounts for 11.7% of new cases worldwide and surpasses lung cancer as the most common malignant tumor (1). At present, the treatment of breast cancer mainly consists of surgical treatment, chemotherapy, endocrine therapy (hormone therapy), targeted therapy, and radiotherapy (2). For example, trastuzumab is the only molecular targeted therapy drug that has a profound impact on Her2-positive breast cancer. The results of a large number of clinical trials have confirmed (3) that the use of trastuzumab adjuvant therapy can reduce the recurrence rate by about 50%. The first-line monotherapy of tocilizumab has a clinical effective rate of about 38%, but drug resistance can occur in about one year of clinical use, and it has limitations such as high price and obvious side effects. Most of these treatments act locally rather than systemically and are thus unable to cure the cancer completely, as cancer is a local manifestation of a systemic disease. In addition, while surgery, radiotherapy, and chemotherapy can kill tumors, they also injure the body’s vital energy. Therefore, the use of natural anti-tumor drugs with low toxicity and effectiveness is an emerging field in tumor treatment.
Muscone (C16H30O), an important component of artificial musk synthesis, is known for its antioxidant, anti-inflammatory, anti-fibrotic, and angiogenic modulating effects (4-6). A study (7) has shown that muscone significantly inhibits the proliferation of drug-resistant cells in lung cancer, and in vivo experiments have confirmed the ability of muscone to inhibit the tumor growth process in mice. One study (8) showed that muscone reduced the expression of vascular endothelial growth factor (VEGF) in mice with “blood stasis” breast cancer and inhibited tumor growth. In another study, the complement component 3 (C3) levels and lymphocyte conversion rates of patients with gastric cancer were significantly higher than those before surgery; in addition, extensive fulminant endolymph node hyperplasia was observed in patients who again passed surgery with muscone, and their lymph node biopsies did not indicate metastatic cancer (9). These studies confirm the significant antitumor effect of muscone. However, the exact molecular mechanism of muscone is still unclear, hindering its further research and clinical guidance of use.
Network pharmacology is a current research frontier in traditional Chinese medicine (TCM). Informed by systems biology and multidirectional pharmacology, network pharmacology aims to uncover the mechanisms of drug action by constructing a complex network between “drug–target–disease”, thus shifting pharmacological research from the traditional search for a single target to a comprehensive network analysis. Network pharmacology analysis can show that the same disease is regulated by different functional genes or proteins at different stages of development, and that some functional proteins play a central regulatory role in multiple diseases. This is similar to the “different treatment for the same disease” and “different treatment for different diseases” theories in TCM. Therefore, the complex chemical composition and multi-target and multi-level pharmacological effects of TCM are naturally compatible with network pharmacology. Network pharmacology can be used to analyze and elaborate the mechanism of action of TCM compounds from a holistic perspective, which is conducive to conducting in-depth research of TCM compounds and expanding their clinical indications. The molecular docking technique is a method to evaluate the interaction between organic small molecule ligands and target proteins. Therefore, on the basis of network pharmacology and molecular docking, the anti-breast cancer mechanism of action of muscone may be discovered for the first time. First, muscone targets associated with breast cancer disease were obtained from the TCMSP and GeneCards databases to establish a dynamic component-target gene network. Next, the STRING database was used to establish the muscone key target protein-protein interaction (PPI) network, and core target genes were screened for survival analysis and validation. Next, Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed for the key targets. Finally, the selected core target genes were molecularly docked to the active ingredients. In this study, seven key target genes affected by musk were initially screened. Through visual network analysis, 10 important signaling pathways of muscone were obtained to explore the key target genes of muscone that exert preventive and curative effects on breast cancer, largely providing a reference of relevant herbal formulations for the treatment of breast cancer.
Methods
Target acquisition of muscone and breast cancer
We obtained the chemical structure of muscone using the PubChem database (https://pubchem.ncbi.nlm.nih.gov), uploaded the structure of the compound to the SwissTargetPrediction database (http://www.swisstargetprediction.ch), and retrieved the target of muscone. We entered the keyword “breast cancer” in the GeneCard database (https://www.genecards.org) and obtained a set of breast cancer–related targets. Finally, we represented the intersection of the muscone target set and the breast cancer target set (i.e., the potential effect of muscone on breast cancer targets) using a Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Potential target PPI network and visualization network data map of muscone acting on breast cancer
We imported the potential breast cancer targets of muscone into the STRING database (https://string-db.org) and drew an interaction map between the potential targets. Next, we used Cytoscape 3.7.1 (https://cytoscape.org/) to create a target PPI network, calculate degree and betweenness centrality, and identify the key breast cancer targets of muscone (10).
GO and KEGG analysis
We imported the core target genes of muscone on breast cancer into the DAVID (https://david.ncifcrf.gov) to obtain the results of the GO and KEGG. The GO classification and enrichment included 3 categories: biological processes (BPs), cellular components (CCs), and molecular functions (MFs). Finally, we used the WeChat website (http://www.bioinformatics.com.cn) to draw bubble maps of the GO enrichment and KEGG signaling pathway enrichment.
Molecular docking study
We retrieved and downloaded the 3D maps of the key target proteins from the Protein Data Bank (PDB; https://www.rcsb.org), downloaded the 2D structures of the 7 key targets from the PubChem database, and then used Chem3D software (PerkinElmer Informatics Inc., Waltham, MA, USA) tool for optimization. Next, we used PyMOL 2.4.0 software (Schrödinger Inc., New York, NY, USA) to dehydrate the compounds and remove the original ligands. Finally, we used the visualization software of AutoDock (Centre for Computational Structural Biology, La Jolla, CA, USA) to perform a complete molecular docking.
Correlation analysis of gene expression levels of core targets and breast cancer prognosis
The prognostic value of core target genes was assessed using the mapping tool Kaplan-Meier (http://kmplot.com/analysis/), which contains basic information on gene expression data and breast cancer survival. Next, the patient samples were divided into 2 groups (high and low expression) by analyzing the overall survival (OS) and relapse-free survival (RFS) of breast cancer patients. We plotted Kaplan-Meier survival analysis based on 95% confidence intervals (CI) and hazard ratios (HR) to analyze core target genes.
Results
Screening of potential targets for the action of muscone acid in breast cancer
We obtained a total of 1,000 target genes related to breast cancer from the GeneCard database and a total of 100 targets related to muscone acid from the SwissTargetPrediction database. Next, we plotted these targets into a Venn diagram, which showed that there were 18 intersecting targets of muscone and breast cancer (Figure 1). These 18 intersecting targets were considered potential targets of muscone for breast cancer (Table 1).
Table 1
| Names | Totals | Elements |
|---|---|---|
| Muscone BRCA | 18 | HSD17B1 |
| NQO2 | ||
| SRD5A2 | ||
| CYP19A1 | ||
| MAPK14 | ||
| CTSB | ||
| GLI1 | ||
| PTGS2 | ||
| TYMS | ||
| MMP1 | ||
| HSP90AA1 | ||
| PARP1 | ||
| ABCG2 | ||
| MMP3 | ||
| PGR | ||
| CYP17A1 | ||
| AR | ||
| MMP9 |
BRCA, breast cancer.
Construction and topological analysis of PPI network of potential targets of muscone
We obtained a PPI map of the potential targets of muscone through the STRING database. The key step was to enter the 18 common drug–disease targets by setting the protein parameter score value to >0.4 in the database (Figure 2A). Next, we imported the network information tab-separated values (TSV) data format into Cytoscape 3.7.1, built a PPI network with the 18 interacting target proteins, and performed a topology analysis. As shown in Figure 2, the node degree values of the target genes AR, PGR, MMP9, PTGS2, HSP90AA1, MAPK14, and CYP19A1 were greater than the average and significantly higher than other target genes. Therefore, we interpreted AR, PGR, MMP9, PTGS2, HSP90AA1, MAPK14, and CYP19A1 as core target genes (Figure 2B and Table 2).
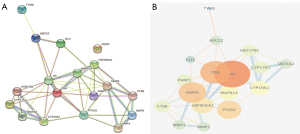
Table 2
| No. | Uniprot | Target gene | Betweenness centrality | Degree |
|---|---|---|---|---|
| 1 | P10275 | AR | 0.29631 | 12 |
| 2 | P06401 | PGR | 0.163671 | 10 |
| 3 | P14780 | MMP9 | 0.138115 | 9 |
| 4 | P35354 | PTGS2 | 0.103333 | 8 |
| 5 | P07900 | HSP90AA1 | 0.029762 | 7 |
| 6 | P16539 | MAPK14 | 0.00375 | 6 |
| 7 | P11511 | CYP19A1 | 0.024028 | 6 |
GO enrichment analysis and KEGG signaling pathway enrichment analysis
A GO enrichment analysis was performed on the 18 potential targets using the DAVID database. We imported the data into the microbiology online tool to plot the GO enrichment analysis bubble plots (Figure 3A-3C) and screened the BPs, CCs, and MFs in the result plots using P<0.05. The results showed that the potential targets were closely related to 32 BPs, 10 CCs, and 18 MFs. The BPs were mainly enriched in steroid biosynthetic process, collagen catabolic process, response to drug, oxidation-reduction process, extracellular matrix, reduction process, extracellular matrix disassembly, positive regulation of brown fat cell differentiation, prostate gland growth, progesterone, progesterone metabolic process, androgen biosynthetic process, estrogen biosynthetic process, proteolysis, positive regulation of protein oligomerization, positive regulation of transcription from RNA polymerase II promoter, positive regulation of vascular smooth muscle, positive regulation of vascular smooth muscle cell proliferation, androgen metabolic process, macrophage differentiation, decidualization, sterol metabolic process, sex differentiation, cell-to-cell signaling, and liver regeneration. The CCs were mainly enriched in the nucleoplasm, protein complex, proteinaceous extracellular matrix, mitochondrion, and neuronal cell. The MFs were mainly enriched in enzyme binding, endopeptidase activity, serine-type endopeptidase activity, endopeptidase activity, endoplasmic reticulum membrane, endopeptidase activity, The MFs were mainly enriched in enzyme binding, endopeptidase activity, serine-type endopeptidase activity, endopeptidase activity, endoplasmic reticulum membrane, endopeptidase activity, zinc ion binding, metalloendopeptidase activity, steroid binding, protein homodimerization activity, sequence-specific DNA binding on the RNA polymerase II core promoter proximal region, protein homodimerization activity, oxygen binding.
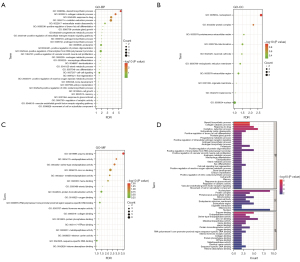
We also performed a KEGG enrichment analysis of the 18 potential targets using the DAVID database. The resultant plots showed that the potential targets were closed linked to 7 signaling pathways (Figure 3D, Table 3), including ovarian steroidogenesis, steroid hormone biosynthesis hormone biosynthesis, pathways in cancer, TNF signaling pathway, progesterone-mediated oocyte maturation, prostate cancer, and bladder cancer (Table 3).
Table 3
| Number | Pathway name | No. of targets | −Log10 P |
|---|---|---|---|
| hsa04913 | Ovarian steroidogenesis | 4 | 3.749024 |
| hsa00140 | Steroid hormone biosynthesis | 4 | 3.530621 |
| hsa05200 | Pathways in cancer | 6 | 2.814438 |
| hsa04668 | TNF signaling pathway | 4 | 2.752475 |
| hsa04914 | Progesterone-mediated oocyte maturation | 3 | 1.771788 |
| hsa05215 | Prostate cancer | 3 | 1.762391 |
| hsa05219 | Bladder cancer | 2 | 1.039475 |
TNF, tumor necrosis factor.
Constructing and analyzing the “component-target-pathway” network of muscone acid in breast cancer
Cytoscape 3.7.1 was used to create a “component-target-pathway” diagram (Figure 4), which showed that the 18 targets and 7 signaling pathways were closely related to each other. Pathways in cancer, ovarian steroidogenesis, and TNF signaling pathway had the most interconnections, with 6, 4, and 4 targets, respectively (Figure 4).
Next, we molecularly docked the muscone components with the key targets AR, PGR, MMP9, PTGS2, HSP90AA1, MAPK14, and CYP19A1 one by one. It is known that lower binding energy scores indicate more stable binding when binding energy indicators in spontaneous ligand-receptor binding less than 0. We performed molecular docking using PyMOL 2.4.0 software and AutoDock’s visualization software (Table 4) and plotted the docking results as a 3D schematic (Figure 5). In addition to the binding energy, the hydrogen bonding of molecular docking was also an important indicator of the degree of flexibility of molecular docking, as shown in Figure 5, where AR, PGR, PTGS2, and MAPK14 all have hydrogen bonds (yellow dashed line). This evidence suggested that muscone might be a key active ingredient in the treatment of breast cancer.
Table 4
| Target | Active ingredients | Binding energy |
|---|---|---|
| AR | Muscone | −6.2 |
| PGR | Muscone | −5.6 |
| MMP9 | Muscone | −6.4 |
| PTGS2 | Muscone | −6.9 |
| HSP90AA1 | Muscone | −6.2 |
| MAPK14 | Muscone | −5.9 |
| CYP19A1 | Muscone | −7 |
Correlation analysis of core target genes and prognosis of patients with breast cancer
To investigate whether the core targets of muscone acting on breast cancer can be used as molecular markers to predict breast cancer, the Kaplan-Meier mapping tool was used to analyze the relationship between the expression levels of seven core target genes and the survival of breast cancer patients (Figure 6). The results showed that high expression of AR, PGR, PTGS2, and HSP90AA1 target genes were associated with survival. OS was significantly higher in patients with low HSP90AA1 expression than in patients with high HSP90AA1 expression (P<0.05). However, the survival of patients with high expression of AR, PGR, and PTGS2 genes was significantly higher than that of patients with low expression of AR, PGR, and PTGS2 genes (P<0.05). MMP9, MAPA14, and CYP19A1 gene expression was not associated with the OS of patients (P>0.05).

Discussion
As the leading cause of cancer in women worldwide (11), breast cancer poses a serious threat to women’s health. Therefore, it is necessary to find safe and effective drugs to inhibit the development and process of breast cancer. Musk has been reported to have good anti-tumor effects, but natural musk is expensive and hard to find, although synthetic musk is different from natural musk in composition, its medicinal value is the same as natural musk. In this study, we investigated the effect of muscone on breast cancer and its mechanism using network pharmacology and molecular docking. We obtained 18 potential targets of muscone acid in breast cancer through databases, constructed a PPI network for the 18 potential targets and performed a topological analysis, and finally obtained 7 key targets according to the degree and mediator value. These targets were AR, PGR, MMP9, PTGS2, HSP90AA1, MAPK14, and CYP19A1. AR is a member of the nuclear hormone receptor family. Composed of three structural domains, the activity of AR transcription factors is activated by the interaction of the ligand-binding domain with the N-terminal regulatory domain in the presence of steroids. Activated ARs are able to form complexes with DNA, and the interaction between regulatory co-blocker and co-activator proteins can stimulate the activity of the complexes. For example, the N-terminal structural domain and the DNA-binding structural domain can mediate the interaction between RAN and BP9 (12,13). A study (14) has shown that positive AR expression predicts higher OS and disease-free survival (DFS) in patients with breast cancer.The progesterone receptor (PGR) is a member of the steroid receptor superfamily. Can participate in the regulation of eukaryotic gene expression, and can also affect cell proliferation and differentiation (15,16). PGR testing remains highly controversial as an important component in assessing breast cancer prognosis and directed treatment planning (17,18). Based on this controversy, Davey et al. (19) evaluated the impact of PGR negativity on tumor outcomes in ER+ breast cancer patients and concluded that PGR negativity independently predicted worse DFS and OS in these patients. Matrix metalloproteinases (MMPs), also known as matrix proteins, are zinc-dependent endopeptidases and major proteases for extracellular matrix degradation. MMPs are able to degrade several extracellular molecules and some bioactive molecules. Matrix metalloproteinases play an important role in local proteolysis of the extracellular matrix and leukocyte migration. MMP9 is a protein-coding gene. Diseases associated with MMP9 include epiphyseal dysplasia 2 and epiphyseal dysplasia (20-22). Expression of MMP9 is significantly associated with breast cancer malignancy, with one study showing that transient overexpression of MMP9 in breast cancer cell lines strongly enhanced malignant features of cells in vitro, such as cell population formation, migration, EMT, and transcription of SMAD (23). Prostaglandin-transsuperoxide synthase 2 (PTGS2) is a protein-coding gene. Dicyclooxygenase and peroxidase in the biosynthetic pathway of prostaglandin, prostaglandin is a class of C20 oxygen lipids mainly derived from arachidone, and has a special role in inflammation. Diseases associated with PTGS2 include gastric ulcers and stomach problems (24). PTGS2 might also have important implications for the diagnosis and prognosis of breast cancer. A study (25) by de Souza et al. found that PTGS2 expression in patients with breast cancer was significantly higher in peripheral blood than in controls. Another study (26) showed that delta-5-desaturase (D5D) knockdown ensured the anti-cancer activity of PTGS2-mediated dihomo-γ-linolenic acid (DGLA) peroxide while promoting the formation of 8-hydroxyoctanoic acid (8-HOA), which could inhibit the growth and metastasis of breast cancer cells and improve the anti-cancer effect of chemotherapy on breast cancer. HSP90AA1 (Heat shock protein 90α class A member 1) is a protein-coding gene. Molecular chaperones capable of promoting the maturation, structural maintenance and proper regulation of specific target proteins, such as those involved in cell cycle control and signal transduction. HSP90AA1 is not only able to interact with specific target proteins and series of target proteins, for example, it is able to promote the maturation of target proteins and participate in cell cycle control and signal transduction (27,28). In our study, HSP90AA1 was highly expressed in breast cancer tissues, and the low expression of HSP90AA1 indicated a good prognosis for patients with breast cancer. Moreover, a study by Liu et al. (29) showed that pre-treatment plasma HSP90AA1 levels in combination with other markers could be an effective means of predicting breast cancer metastasis rates. MAPK14 is one of the four p38 MAPKs and a member of the MAP kinase family. MAP kinases are integration points for a variety of biochemical signals and are normally capable of participating in cell proliferation, differentiation, transcriptional regulation and development. This kinase is activated by various environmental stresses and pro-inflammatory cytokines. MAPK14 plays an important role in cellular responses to extracellular stimuli, such as the direct activation of transcription factors by pro-inflammatory cytokines. Thus, p38 MAPKs lead to phosphorylation of proteins (30). MAPK14 is significantly expressed in tumor tissues and has a predictable impact on clinical features and is associated with the immune microenvironment of colorectal cancer (31). The CYP19A1 gene is a member of the cytochrome P450 superfamily of enzymes. It can catalyze many lipid reactions, such as drug metabolism and cholesterol and steroids. C19 androgens, androst-4-ene-3,17-dione (androstenedione) and testosterone can be catalyzed by CYP19A1, and all three are converted to C18 estrogens, estrone and estradiol, respectively (32-34). Patients with breast, endometrial, or ovarian malignancies exhibit high levels of CYP19A1 and ER, with high expression of CYP19A1 indicating a poorer prognosis for patients (35).
Our GO and KEGG analysis of potential targets of muscone action in breast cancer showed that muscone regulates signaling pathways (cancer pathways, TNF signaling, ovarian steroidogenesis, and steroid hormone biosynthesis) through molecular responses (steroid biosynthesis, collagen catabolism, response to drugs, redox processes, extracellular matrix breakdown, positive regulation of brown adipocyte differentiation, prostatic hyperplasia, progesterone metabolism, positive intracellular estrogen receptor signaling regulation, androgen biosynthesis process, estrogen biosynthesis process), thereby exerting the inhibitory effect of muscone on breast cancer cell. The KEGG signaling pathway enrichment analysis revealed that AR, HSP90AA1, PTGS2, and MMP9 were all enriched in cancer pathways. MAPK14, PTGS2, and MMP9 were all enriched in the TNF signaling pathway, and muscone and breast cancer were associated with the regulation of cancer pathways and TNF. Therefore, we predict that these 2 pathways could be the molecular mechanism for the efficacy of muscone acid in breast cancer. However, the research done in this paper only provides some new ideas for the selection of prescriptions and drugs. The prescriptions for the treatment of breast cancer also need to rely on doctors with rich clinical experience, and clinical trials and basic experiments are needed to further prove.
Molecular docking, as a theoretical simulation method to study ligand-receptor interaction and predict its binding mode and affinity, is mainly a drug design method based on receptor characteristics and the interaction mode between receptor and target compound molecules. Our results showed that muscone had good binding activity with core target genes and that muscone might exert anti-breast cancer effects by binding core target genes.
The Kaplan-Meier plotter tool is an online analytical database of prognostic relevance in malignancies that allows the assessment of the impact of more than 54,000 genes on the prognosis of 21 cancer types. We used the Kaplan-Meier plotter tool to analyze the survival curves of 7 core target genes of muscone action in breast cancer. The results showed that patients with low expression of HSP90AA1 had significantly higher survival than patients with high expression of HSP90AA1 (P<0.05), while patients with high expression of AR, PGR, and PTGS2 had significantly higher survival than patients with low expression of AR, PGR, and PTGS2 (P<0.05). Therefore, HSP90AA1, AR, PGR, and PTGS2 target genes might be an observable indicator of breast cancer survival prognosis.
By predicting the relationship between the components of traditional Chinese medicine and disease-related genes and targets, network pharmacology reveals the regulatory effect of traditional Chinese medicine components on major enrichment pathways through core compounds and genes, and explains the mechanism of action of traditional Chinese medicine on diseases. Our research mainly focuses on the mechanism of action of muscone on breast cancer, aiming to provide an effective scientific basis for muscone in the treatment of breast cancer. However, this study has not been verified by in vivo experiments, so there is a lack of experimental data to support it. It is of great significance to conduct more systematic research on muscone in the future to explore its detailed mechanism.
Conclusions
This study uses a network pharmacology approach and molecular docking techniques to investigate the effects of muscone’s mechanism of action on breast cancer through complex analysis of targets and pathways. The results showed that muscone could treat breast cancer through multiple targets and pathways. Core target targets such as AR, PGR, MMP9, PTGS2, HSP90AA1, MAPK14, and CYP19A1 were involved in molecular reactions such as steroid biosynthesis process, collagen catabolic process, response to drugs, oxidative reduction process etc., which might in turn exert anti-breast cancer effects. However, the study has some limitations, because the study is only theoretical and no experimental validation has been performed. Later we will validate it by mouse experiments, and if the theoretical study is proven, new drugs for breast cancer treatment can be researched based on the obtained pathways and the molecular processes involved, and a breakthrough in breast cancer treatment may be obtained.
Acknowledgments
Funding: The authors disclose that the research, authorship, and/or publication of this article was financially supported by the following:
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-667/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Trayes KP, Cokenakes SEH. Breast Cancer Treatment. Am Fam Physician 2021;104:171-8. [PubMed]
- Zhang Y, Liu XF, Wang N, et al. Effects of Yanghe Huayan Decoction combined with Trastuzumab on PTEN-PI3K/Akt pathway and VEGFC in HER-2 overexpressing breast cancer cells. Jiangsu Journal of Traditional Chinese Medicine 2018;50:79-82. [Crossref]
- Du Y, Ge Y, Xu Z, et al. Hypoxia-Inducible Factor 1 alpha (HIF-1α)/Vascular Endothelial Growth Factor (VEGF) Pathway Participates in Angiogenesis of Myocardial Infarction in Muscone-Treated Mice: Preliminary Study. Med Sci Monit 2018;24:8870-7. [Crossref] [PubMed]
- Kailiang Z, Yihui Z, Dingsheng L, et al. Effects of Muscone on Random Skin Flap Survival in Rats. J Reconstr Microsurg 2016;32:200-7. [Crossref] [PubMed]
- Du Y, Gu X, Meng H, et al. Muscone improves cardiac function in mice after myocardial infarction by alleviating cardiac macrophage-mediated chronic inflammation through inhibition of NF-κB and NLRP3 inflammasome. Am J Transl Res 2018;10:4235-46. [PubMed]
- Lu P, Fan JJ, Luo X, et al. The effect of Muscone on lung cancer cells resistance to cisplatin and tumor growth in mice. Journal of Guangxi Medical University 2020;37:1948-53.
- Liu YH, Chang J, Xue LJ, et al. Effects of musk ketone on the growth of blood stasis breast cancer model and expression of VEGF. Journal of Xi’an Jiaotong University (Medical Sciences) 2014;35:547-50.
- Meng ZH, Ding KB, Wang WC, et al. Long-term results of burying musk in the abdomen for prolonging the survival of gastric cancer (Report of 74 cases). Medical Journal of Chinese People's Liberation Army 1993;18:303-5.
- Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13:2498-504. [Crossref] [PubMed]
- Lima SM, Kehm RD, Terry MB. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine 2021;38:100985. [Crossref] [PubMed]
- Venema CM, Bense RD, Steenbruggen TG, et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol Ther 2019;200:135-47. [Crossref] [PubMed]
- Hsu CL, Liu JS, Wu PL, et al. Identification of a new androgen receptor (AR) co-regulator BUD31 and related peptides to suppress wild-type and mutated AR-mediated prostate cancer growth via peptide screening and X-ray structure analysis. Mol Oncol 2014;8:1575-87. [Crossref] [PubMed]
- Venema CM, Bense RD, Steenbruggen TG, et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol Ther 2019;200:135-47. [Crossref] [PubMed]
- Olivotto IA, Truong PT, Speers CH, Bernstein V, Allan SJ, Kelly SJ, Lesperance ML. Time to stop progesterone receptor testing in breast cancer management. J Clin Oncol. 2004;22:1769-70. [Crossref] [PubMed]
- Olivotto IA, Truong PT, Speers CH, Bernstein V, Allan SJ, Kelly SJ, Lesperance ML. Time to stop progesterone receptor testing in breast cancer management. J Clin Oncol. 2004;22:1769-70. [Crossref] [PubMed]
- Colomer R, Beltran M, Dorcas J, et al. It is not time to stop progesterone receptor testing in breast cancer. J Clin Oncol 2005;23:3868-9; author reply 3869-70. [Crossref] [PubMed]
- Olivotto IA, Truong PT, Speers CH, et al. Time to stop progesterone receptor testing in breast cancer management. J Clin Oncol 2004;22:1769-70. [Crossref] [PubMed]
- Davey MG, Ryan ÉJ, Folan PJ, et al. The impact of progesterone receptor negativity on oncological outcomes in oestrogen-receptor-positive breast cancer. BJS Open 2021;5:zrab040.
- Takino T, Koshikawa N, Miyamori H, et al. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene 2003;22:4617-26. [Crossref] [PubMed]
- Ortega N, Wang K, Ferrara N, et al. Complementary interplay between matrix metalloproteinase-9, vascular endothelial growth factor and osteoclast function drives endochondral bone formation. Dis Model Mech 2010;3:224-35. [Crossref] [PubMed]
- Landino LM, Crews BC, Gierse JK, et al. Mutational analysis of the role of the distal histidine and glutamine residues of prostaglandin-endoperoxide synthase-2 in peroxidase catalysis, hydroperoxide reduction, and cyclooxygenase activation. J Biol Chem 1997;272:21565-74. [Crossref] [PubMed]
- Dong H, Diao H, Zhao Y, et al. Overexpression of matrix metalloproteinase-9 in breast cancer cell lines remarkably increases the cell malignancy largely via activation of transforming growth factor beta/SMAD signalling. Cell Prolif 2019;52:e12633. [Crossref] [PubMed]
- Musee J, Marnett LJ. Prostaglandin H synthase-2-catalyzed oxygenation of 2-arachidonoylglycerol is more sensitive to peroxide tone than oxygenation of arachidonic acid. J Biol Chem 2012;287:37383-94. [Crossref] [PubMed]
- de Souza CP, Alves B, Waisberg J, et al. Detection of COX-2 in liquid biopsy in patients with breast cancer. J Clin Pathol 2020;73:826-9. [Crossref] [PubMed]
- Xu Y, Yang X, Wang T, et al. Knockdown delta-5-desaturase in breast cancer cells that overexpress COX-2 results in inhibition of growth, migration and invasion via a dihomo-γ-linolenic acid peroxidation dependent mechanism. BMC Cancer 2018;18:330. [Crossref] [PubMed]
- Martínez-Ruiz A, Villanueva L, González de Orduña C, et al. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci U S A 2005;102:8525-30. [Crossref] [PubMed]
- Woodford MR, Sager RA, Marris E, et al. Tumor suppressor Tsc1 is a new Hsp90 co-chaperone that facilitates folding of kinase and non-kinase clients. EMBO J 2017;36:3650-65. [Crossref] [PubMed]
- Liu H, Zhang Z, Huang Y, et al. Plasma HSP90AA1 Predicts the Risk of Breast Cancer Onset and Distant Metastasis. Front Cell Dev Biol 2021;9:639596. [Crossref] [PubMed]
- Ondet T, Muscatelli-Groux B, Coulouarn C, et al. The release of pro-inflammatory cytokines is mediated via mitogen-activated protein kinases rather than by the inflammasome signalling pathway in keratinocytes. Clin Exp Pharmacol Physiol 2017;44:827-38. [Crossref] [PubMed]
- Wang D, Peng L, Hua L, et al. Mapk14 is a Prognostic Biomarker and Correlates with the Clinicopathological Features and Immune Infiltration of Colorectal Cancer. Front Cell Dev Biol 2022;10:817800. [Crossref] [PubMed]
- Corbin CJ, Graham-Lorence S, McPhaul M, et al. Isolation of a full-length cDNA insert encoding human aromatase system cytochrome P-450 and its expression in nonsteroidogenic cells. Proc Natl Acad Sci U S A 1988;85:8948-52. [Crossref] [PubMed]
- Sohl CD, Guengerich FP. Kinetic analysis of the three-step steroid aromatase reaction of human cytochrome P450 19A1. J Biol Chem 2010;285:17734-43. [Crossref] [PubMed]
- Cheng Q, Sohl CD, Yoshimoto FK, Guengerich FP. Oxidation of dihydrotestosterone by human cytochromes P450 19A1 and 3A4. J Biol Chem 2012;287:29554-67. [Crossref] [PubMed]
- Yang T, Wu WJ, Tian LM, et al. The Associations of Androgen-Related Genes CYP21A2 and CYP19A1 with Severe Acne Vulgaris in Patients from Southwest China. Clin Cosmet Investig Dermatol 2021;14:313-31. [Crossref] [PubMed]
(English Language Editor: C. Gourlay)





