
Radiation induced lung injury (RILI) after postoperative intensity modulated proton therapy (IMPT) in a patient with stage III locally advanced lung adenocarcinoma: a case report
Introduction
Albeit multiple curative strategies have been involved in the treatment of lung cancer, it is still the primary cause of cancer associated mortality. Non-small cell lung cancer (NSCLC) is the main subtype of lung cancer, among which lung adenocarcinoma is the most common form. Surgery is the main treatment for the resectable stage III-pN2 NSCLC, but local recurrence and distant metastasis still occur frequently after surgery. It has been demonstrated that postoperative chemotherapy can reduce distant metastasis for patients with positive lymph nodes. However, the risk of locoregional recurrence reached as evidently as 20–40% even after complete resection, adjuvant and postoperative chemotherapy (1,2). Postoperative radiation therapy (PORT) improved the locoregional recurrence survival (LRFS) in resected pIIIa-N2 NSCLC (3). Thus, postoperative chemoradiation remains critical in the stage III NSCLC.
Radiation induced lung injury (RILI), manifesting as radiation pneumonitis acutely and radiation pulmonary fibrosis chronically, limits the dose escalation and negatively affects survival and quality of life. Distinguished from photons, the track of protons’ motion is characterized with the Bragg peak, depositing a high irradiation dose in the tumor, while leaving a negligible dose in the adjacent normal tissues. Thereafter, proton therapy (PT) has emerged as a valuable radiotherapy modality that can improve treatment outcomes (4).
Passive scattering proton therapy (PSPT) is a previous PT technology. PSPT irradiates tumor spot by spot and deposits a homogenous dose to tumor, which is similar to three-dimension radiation plan. Intensity modulated proton therapy (IMPT) shapes tumor better to deliver a more flexible and conformal dose distributions compared with PSPT (5,6). Past study demonstrated that PT could minimize the risk of RILI by sparing organ-at-risk (OAR) dose compared with conventional photon radiation therapy (RT) (7). Moreover, patients with idiopathic pulmonary fibrosis and recurrences of previous irradiated tissues were proved to benefit from PT instead of photon RT with less occurrence of RILI (8). The rate of pulmonary toxicities of IMPT was lower than 5% according to the past studies (1,9). Lung injury with fibrosis formation is not commonplace in the condition where conservative dose of IMPT delivered in the patient without chronic lung disease (10). Here, we report RILI induced by IMPT sequentially following adjuvant chemotherapy in a patient with locally advanced lung adenocarcinoma. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-256/rc).
Case presentation
In August 2020, a 47-year-old man with a history of heavy tobacco usage accidentally found a 1.5 cm pulmonary nodule on left lower lobe (LLL) by computed tomography (CT) in our hospital. The patient had no pulmonary disease before but had been diagnosed with diabetes mellitus (DM). Other radiographic evaluations showed no evidence of distant metastasis at that time. Then he underwent a video-assisted left lower pulmonary lobectomy and mediastinal lymph node dissection on December 3, 2020. Postoperative biopsy established the pathologic diagnosis of lung acinar-predominant adenocarcinoma (pT1bN2M0, stage IIIA). The invasive nodule was 16 mm, spreading along the air cavity, but had no breakthrough to the adjacent pleura. The broken end of bronchus and left pulmonary hilar vessels were tumor free. Lymph nodes stations (#4, #5, #7, #9, #10, #11, #12) were excised and the subcarinal (#7) and hilar (#10) were tumor positive. Molecular detection showed fusion of rearranged during transfection (RET) gene. Other common oncogenes including epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), KRAS proto-oncogene (KRAS), NRAS proto-oncogene (NRAS), human epidermal growth factor receptor 2 (HER2), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), B-Raf proto-oncogene (BRAF) had no alternations.
The patient underwent postoperative adjuvant chemotherapy with AP (pemetrexed 1,000 mg d2 + carboplatin 500 mg d1, q21d) for 6 cycles and accomplished the therapy on April 28, 2021. CT after 4 cycles indicated the lungs were in well condition and the tumor had no recurrence (Figure 1A). Sequentially, on May 7 to June 9, 2021, the patient received IMPT in Shanghai. Four dimentional (4D) CT was applied for visual simulation to manage the respiratory changes. The clinical target volume (CTV) encompassed the resection involved anatomical mediastinal lymph node regions, the bronchial stump, the ipsilateral hilum, #4 and #7 nodal stations in the mid lung according to 2018 ESTRO ACROP guidelines (11). The final planning target volume (PTV) was delineated by adding a 5-mm margin to the CTV boundary to adjust for setup uncertainties. A total of 50 Gy (RBE) in 25 fractions was delivered to the PTV with the robust optimization to manage uncertainties in the treatment plan. To quantify the anatomical changes, the imaging feedback loop by cone beam CT (CBCT) was applied to modify the treatment plan accordingly every week. The parameters of IMPT are listed in Table 1.
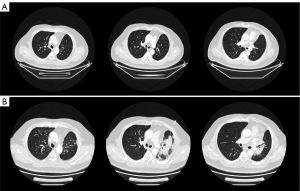
Table 1
| Variables | Values |
|---|---|
| Lung V5 (%) | 28 |
| Lung V20 (%) | 16 |
| MLD (GyE) | 7.44 |
| MHD (GyE) | 2.20 |
| MED (GyE) | 11.45 |
| Lung max (GyE) | 56.06 |
| Heart max (GyE) | 55.00 |
| Eso max (GyE) | 51.97 |
| SC max (GyE) | 11.91 |
IMPT, intensity modulated proton therapy; OARs, organ-at-risks; Lung V5, the lung volume receiving a dose of more than 5 GyE; Lung V20, the lung volume receiving a dose of more than 20 GyE; MLD, mean lung dose; MHD, mean heart dose; MED, mean esophageal dose; max, maximum dose; Eso, esophagus; SC, spinal cord.
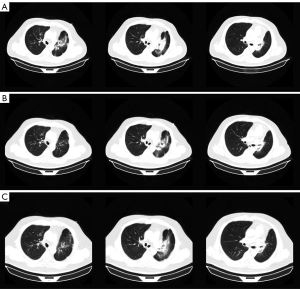
About 8 weeks after IMPT, mild dry cough and exertional dyspnea arose soundlessly. Routine laboratory examination revealed normal count of white blood cells [WBC 7.41×109/L, normal limits (3.5–9.5)×109/L], lymphocytes [LYMPH 1.40×109/L, normal limits (1.1–3.2)×109/L], neutrophils [NEUT 5.29×109/L, normal limits (1.8–6.3)×109/L]. Liver function showed abnormal aspartate aminotransferase (AST 53 IU/L, normal limits 0–42 IU/L), alanine aminotransferase (ALT 80 IU/L, normal limits 0–40 IU/L), and gamma-glutamyl transpeptidase (GGT 58 IU/L, normal limits 0–52 IU/L) . The liver enzymes were elevated since the last time of chemotherapy and it was gradually falling back with the hepatic protectant. High sensitivity C-reactive protein was 9.16 mg/L (HSCRP was 5.03 mg/L in 26th April, normal limits 0–3 mg/L). Carcino-embryonic antigen (CEA) was at the normal level. Etiology examination of phlegm revealed no evidence of pathogen infections. CT on July 30 showed new pathological changes in the left upper lobe (LUL), manifesting as focal dense consolidation accompanied by ground-glass opacities (Figure 1B). The pneumonitis involved 22.1% volume of LUL which was matched with the high dose distribution of the proton (Figure S1). Besides, a 2 cm ground glass opacity in the peripheral right upper lobe (RUL) was new (Figure S2A-S2D). According to the clinical history of IMPT and imaging changes of acute pneumonitis, we attributed the suddenly increased mass to the early phase of RILI. The pneumonitis manifested as patchy focal consolidation and caused moderate symptoms and was classified as grade 2 RILI according to Radiation Therapy Oncology Group (RTOG) grade and Common Terminology Criteria for Adverse Events (CTCAE) (12). According to the management of RILI, steroid therapy is the mainstay of the treatment of RILI. We informed the patient the risk of symptoms worsening in the condition of steroids abrupt interruption (13) and got the agreement of the patient for the continuous treatment. The initial administration of 4.5 mg dexamethasone (equal to 30 mg prednisolone) for 18 days was started on August 1. The reexamination after steroid therapy on 17th August indicated that the involvement filed of pneumonitis decreased a little and the margins got sharper (Figure 2A). The dexamethasone was then adjusted to 6 mg for 9 days. However, the consolidation on LUL didn’t attenuate ideally on 24th August (Figure 2B). Therefore, the intravenous injection of methylprednisolone with a dose of 80 mg (equal to 100 mg prednisolone) per day was used for 4 days from 27th to 31st August. Then the steroids were changed back to 6 mg dexamethasone per day on September 5 and followed by reduction of 0.75 mg every week. The patient felt the symptomatic relief with reduction in cough and dyspnea. CT indicated that most of the consolidation vanished with a fraction of scar which changed slightly on 3rd, 14th September, 25th October, 20th December (Figure 2C, Figure S3). Based on the slight radiographic changes, the grade 3 radiation fibrosis had formed according to radiological grading scale of radiation induced pneumonitis (14). The total period of steroid therapy was more than 12 weeks, but the radiographic amelioration was slow. The long period of treatment indicated that RILI after IMPT was somehow different from conventional RILI for the potential resistance to steroids. Prophylactic antibiotic was used for the steroids induced immunosuppression. No adverse events occurred during the treatment of RILI. The timeline was shown in Figure 3. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
RILI is a major dose-limiting toxicity induced by thoracic radiotherapy. Free radicals deriving from water in irradiated tissues break chemical bonds of DNA to destroy epithelial and endothelial cells, leading to RILI. There are two phases in the RILI. Acute phase, which is known as radiation pneumonitis, occurs within 6 months of the therapy. Chronic phase always manifests as irreversible lung fibrosis and develops late in 6 months after radiation (15). The hyper-acute phase and latent phase, which are initiation of cytokine release waves, occur within 2 weeks after radiation asymptomatically. Radiographic changes can’t be found in the two phases. The acute exudative stage lasts from 3 weeks to 4 months after radiation. Ground-glass attenuation and patchy consolidation can be seen in the phase. The initiation of profibrogenic begins from 4 months after radiation. The consolidation tends to be scarring and changes little after steroid treatment. Generally, lung fibrosis generates after 6months. Steroids are mainstay for RILI, the course of treatment is 3–12 weeks and usually terminated within 12 weeks.
Despite more sophisticated techniques of conventional photon radiotherapy such as intensity modulated radiotherapy (IMRT) and stereotactic body radiotherapy (SBRT), the incidence of RILI was about 5–25% in lung cancer (11). PT emerges as a new-style RT with accurate distribution of curative dose to tumor. Theoretically, PT provides superior dose distributions vs photon therapy by increasing the OAR sparing and protecting adjacent tissues from extra radiation injury. However, PSPT did not confer a clinical benefit of reducing RP compared with IMRT (2). IMPT is dosimetrically superior to PSPT and associated with lower radiation doses to the lung and heart. According to past studies, the pulmonary toxicities rates of PSPT was about 20% (16). When it came to IMPT, the rate was lower than 5%. In the retrospective review of 136 PORT patients, rates of higher than grade 2 pneumonitis were 17.0% in IMRT cohort and 4.9% in the PT cohort (both PSPT and IMPT) (1). In the recent comparative study of local advanced NSCLC treated with concurrent chemotherapy and proton beam therapy, IMPT showed lower rates of pulmonary toxicities (28% versus 3%, P=0.006) (9). The results proved that lung injury in IMPT was not a commonplace.
RILI is related to radiation dose. Doses of 45 to 54 Gy in 1.8 to 2 Gy fractions are standard preoperative doses in NSCLC according to National Comprehensive Cancer Network (NCCN) guidelines (17). PT achieved escalated dose with excellent survival and minimal toxicity (18). In the previous study of IMPT, most of patients (96.8%) patients in the IMPT cohort received total dose of more than 50 Gy (RBE) with the 4.9% incidence of grade 2 and the more severe pneumonitis (1). A reasonable and conservative radiation dose was delivered in the case so that there was no evidence for the dose related RILI.
Despite the control of uncertainties from intrafield target motion due to unavoidable respiration and cardiac motion and daily setup anatomical changes were managed ahead of schedule during the thoracic radiation treatment planning, IMPT is highly sensitive to the slight difference since proton pencil beam delivers different relative linear stopping powers at the path of moving into and out of the tissue due to the slight change (19,20). Daily adaptive optimization before deliver each fraction is pre-clinical to guarantee a high accuracy but is limited to the time consuming (21). Besides 4DCT, passive breath hold is a promising strategy for the motion mitigation (22).
Past review demonstrated that elder patients, women with a lower lung volume, patients with comorbid conditions [such as chronic obstructive pulmonary disease and interstitial lung disease (ILD)] were always at the risk of RILI (23). However, our patient was a 47-year-old strong man without chronic pulmonary disease. Patients with a history of diabetes are more likely to suffer from severe RILI based on the analysis in vivo. Hyperglycemia improves levels of chronic inflammatory factors such as interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α) by activating nuclear factor kappa-B (NF-κB) through receptor for advanced glycation end-products (RAGE). The cytokine cascade can thereafter stimulate collagen synthesis in fibroblasts, leading to RILI (24). Nevertheless, the effect of diabetes on RILI is far from clear and lack of clinical evidence.
It was reported that pre-RT radio-sensitizing chemotherapy increased the risk of RILI, especially the combination of chemotherapeutic agents such as taxanes, vincristine and bevacizumab, which are commonly used in NSCLC (25). The incidence of pemetrexed related ILD was 1.8–2.6% in NSCLC (26). It was once reported a 69 man with stage IV lung adenocarcinoma who developed ILD after the second line of pemetrexed (27). However, drug induced ILD always manifest as bilateral lung infiltrates, ground-glass opacity (GGO), alveolar hemorrhage, alveolitis and occur in subacute form after the drug administration (28). The clinical features of our case didn’t conform with drug induced ILD obviously. Nevertheless, the pre-IMPT chemotherapy might not get rid of responsibility. Although there was no analysis about the effect of interval of chemoradiotherapy on RILI, we speculated that the short interval might make sense. The interval of chemotherapy and PORT was usually within the 45 days but more than 26 days (29). However, the interval in this case was less than 10 days, which might be a potential risk factor. Observation and comparative studies should fill in the blank to choose the optimal time for radiotherapy sequentially after chemotherapy.
Cytokines including IL-18, IL-1, IL-6, transforming growth factor β (TGF-β), type I interferon secreted by infiltrating cell components are pro-fibrosis drivers (30). Elevated serum levels of the cytokines were potentially predictive for the development of RILI. Alternations in vital genes involving in inflammation and fibrogenesis may play a potential role in the susceptibility to RILI. Single nucleotide polymorphism (SNP) and epigenetic regulation of TGF-β was reported as one of the popular genetic markers of elevated risk of fibrosis following radiotherapy (31). Nevertheless, cellular and molecular biomarkers in predicting RILI are far from applying clinical use. Although we had no idea about cytokine level of the patient, further exploration about molecular biomarkers could be studied in the future.
In spite of only one case, somehow differences in the case were noticed compared with the RILI we treated before. Although steroid therapy was actively used since the first discover of RILI, the consolidation changed slightly and fibrogenesis still occurred. According to the clinical features of chronic RILI described before, lung fibrosis universally generates after 6 months (12). The prolonged taking effect time of steroids could be due to an earlier happening fibrosis phase of RILI.
A few limitations are associated with the study. Firstly, although the IMPT induced lung injury reported by us was indeed refractory and not sensitive to steroids with early developed lung fibrosis, observation studies about the clinical features of proton induced lung injury are needed. Besides, we had no idea about the time of initiation of RILI since the earliest CT was conserved about 7 weeks after IMPT. Moreover, the bronchoscopy or percutaneous biopsy was exempted in the case since the diagnosis of RILI was not complicated.
In this case, we report IMPT induced lung injury in a young patient with stage III locally advanced lung adenocarcinoma. IMPT is superior to PSPT and IMRT with lower radiation doses to the lung for the rapid decay in the adjacent normal tissues and the sophisticated technique, yet RILI is a burning question for its effect on the clinical outcome and quality of life. RILI after IMPT is not commonplace especially under the circumstance where the patient had no chronic lung disease and the proton dose was conservative. Although active steroid therapy was applied, the radiographic changes were inconspicuously and lung fibrosis was developing without pausing. Based on the case, we suggested more exploration of proton induced lung injury and evaluation before IMPT especially following chemotherapy are deserved.
Patient perspective
This is a young patient without chronic pulmonary disease, he chose IMPT for its lower incidence of lung injury as reported before. We had no idea about why he developed RILI despite many speculations had been proposed. The lung fibrosis developed in the condition of active steroid therapy. He would like to alert clinical oncologists and patients to attach more attention to the adverse event by IMPT.
Acknowledgments
We would like to thank the patient and his wife for giving their consent for the publication of this case report and sharing his radiation plan with us.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-256/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-256/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boyce-Fappiano D, Nguyen QN, Chapman BV, et al. Single Institution Experience of Proton and Photon-based Postoperative Radiation Therapy for Non-small-cell Lung Cancer. Clin Lung Cancer 2021;22:e745-55. [Crossref] [PubMed]
- Kim N, Noh JM, Lee W, et al. Clinical Outcomes of Pencil Beam Scanning Proton Therapy in Locally Advanced Non-Small Cell Lung Cancer: Propensity Score Analysis. Cancers (Basel) 2021;13:3497. [Crossref] [PubMed]
- Hui Z, Men Y, Hu C, et al. Effect of Postoperative Radiotherapy for Patients With pIIIA-N2 Non-Small Cell Lung Cancer After Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial. JAMA Oncol 2021;7:1178-85. [Crossref] [PubMed]
- Ramella S, D'Angelillo RM. Proton beam or photon beam radiotherapy in the treatment of non-small-cell lung cancer. Lancet Oncol 2020;21:873-5. [Crossref] [PubMed]
- Chiang JS, Yu NY, Daniels TB, et al. Proton beam radiotherapy for patients with early-stage and advanced lung cancer: a narrative review with contemporary clinical recommendations. J Thorac Dis 2021;13:1270-85. [Crossref] [PubMed]
- Lazarev S, Rosenzweig K, Samstein R, et al. Where are we with proton beam therapy for thoracic malignancies? Current status and future perspectives. Lung Cancer 2021;152:157-64. [Crossref] [PubMed]
- Chi A, Chen H, Wen S, et al. Comparison of particle beam therapy and stereotactic body radiotherapy for early stage non-small cell lung cancer: A systematic review and hypothesis-generating meta-analysis. Radiother Oncol 2017;123:346-54. [Crossref] [PubMed]
- Kim H, Pyo H, Noh JM, et al. Preliminary result of definitive radiotherapy in patients with non-small cell lung cancer who have underlying idiopathic pulmonary fibrosis: comparison between X-ray and proton therapy. Radiat Oncol 2019;14:19. [Crossref] [PubMed]
- Gjyshi O, Xu T, Elhammali A, et al. Toxicity and Survival After Intensity-Modulated Proton Therapy Versus Passive Scattering Proton Therapy for NSCLC. J Thorac Oncol 2021;16:269-77. [Crossref] [PubMed]
- Hashimoto S, Iwata H, Hattori Y, et al. Outcomes of proton therapy for non-small cell lung cancer in patients with interstitial pneumonia. Radiat Oncol 2022;17:56. [Crossref] [PubMed]
- Nestle U, De Ruysscher D, Ricardi U, et al. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother Oncol 2018;127:1-5. [Crossref] [PubMed]
- Rahi MS, Parekh J, Pednekar P, et al. Radiation-Induced Lung Injury-Current Perspectives and Management. Clin Pract 2021;11:410-29. [Crossref] [PubMed]
- Giridhar P, Mallick S, Rath GK, et al. Radiation induced lung injury: prediction, assessment and management. Asian Pac J Cancer Prev 2015;16:2613-7. [Crossref] [PubMed]
- Kouloulias V, Zygogianni A, Efstathopoulos E, et al. Suggestion for a new grading scale for radiation induced pneumonitis based on radiological findings of computerized tomography: correlation with clinical and radiotherapeutic parameters in lung cancer patients. Asian Pac J Cancer Prev 2013;14:2717-22. [Crossref] [PubMed]
- Hanania AN, Mainwaring W, Ghebre YT, et al. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019;156:150-62. [Crossref] [PubMed]
- Ohnishi K, Ishikawa H, Nakazawa K, et al. Long-term outcomes of high-dose (74 GyE) proton beam therapy with concurrent chemotherapy for stage III nonsmall-cell lung cancer. Thorac Cancer 2021;12:1320-7. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer. Version 7. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf
- Ion Beam Applications (IBA). Treating thoracic cancers with proton therapy, current practices, opportunities and challenges. Available online: https://www.iba-worldwide.com/sites/protontherapy/files/media_document/lung_white_paper_final_clean.pdf
- Prasanna PG, Rawojc K, Guha C, et al. Normal Tissue Injury Induced by Photon and Proton Therapies: Gaps and Opportunities. Int J Radiat Oncol Biol Phys 2021;110:1325-40. [Crossref] [PubMed]
- Jongen A, Charlier F, Baker K, et al. Clinical Outcomes After Proton Beam Therapy for Locally Advanced Non-Small Cell Lung Cancer: Analysis of a Multi-institutional Prospective Registry. Adv Radiat Oncol 2021;7:100767. [Crossref] [PubMed]
- Nenoff L, Matter M, Amaya EJ, et al. Dosimetric influence of deformable image registration uncertainties on propagated structures for online daily adaptive proton therapy of lung cancer patients. Radiother Oncol 2021;159:136-43. [Crossref] [PubMed]
- Emert F, Missimer J, Eichenberger PA, et al. Enhanced Deep-Inspiration Breath Hold Superior to High-Frequency Percussive Ventilation for Respiratory Motion Mitigation: A Physiology-Driven, MRI-Guided Assessment Toward Optimized Lung Cancer Treatment With Proton Therapy. Front Oncol 2021;11:621350. [Crossref] [PubMed]
- Arroyo-Hernández M, Maldonado F, Lozano-Ruiz F, et al. Radiation-induced lung injury: current evidence. BMC Pulm Med 2021;21:9. [Crossref] [PubMed]
- Dong G, Li Y, Zhao Q, et al. Effects of diabetes on the development of radiation pneumonitis. Respir Res 2021;22:160. [Crossref] [PubMed]
- Mao J, Kocak Z, Zhou S, et al. The impact of induction chemotherapy and the associated tumor response on subsequent radiation-related changes in lung function and tumor response. Int J Radiat Oncol Biol Phys 2007;67:1360-9. [Crossref] [PubMed]
- Tomii K, Kato T, Takahashi M, et al. Pemetrexed-related interstitial lung disease reported from post marketing surveillance (malignant pleural mesothelioma/non-small cell lung cancer). Jpn J Clin Oncol 2017;47:350-6. [Crossref] [PubMed]
- Kim KH, Song SY, Lim KH, et al. Interstitial Pneumonitis after Treatment with Pemetrexed for Non-small Cell Lung Cancer. Cancer Res Treat 2013;45:74-7. [Crossref] [PubMed]
- Distefano G, Fanzone L, Palermo M, et al. HRCT Patterns of Drug-Induced Interstitial Lung Diseases: A Review. Diagnostics (Basel) 2020;10:244. [Crossref] [PubMed]
- Robinson CG, Patel AP, Bradley JD, et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol 2015;33:870-6. [Crossref] [PubMed]
- Jarzebska N, Karetnikova ES, Markov AG, et al. Scarred Lung. An Update on Radiation-Induced Pulmonary Fibrosis. Front Med (Lausanne) 2021;7:585756. [Crossref] [PubMed]
- Grossberg AJ, Lei X, Xu T, et al. Association of Transforming Growth Factor β Polymorphism C-509T With Radiation-Induced Fibrosis Among Patients With Early-Stage Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol 2018;4:1751-7. [Crossref] [PubMed]



