
Efficacy and safety of anlotinib in metastatic adenoid cystic carcinoma: a retrospective study
Introduction
Adenoid cystic carcinoma (ACC) is a rare kind of malignancy commonly arising in salivary glands and occasionally originating in other sites, such as lung, trachea and breast (1). ACC is characterized by unpredictable clinical course, extensive perineural invasion and high risk of metastasis. Treatment remains limited to local treatment such as surgery and adjuvant radiotherapy. Even if the primary tumor could be controlled, distant metastatic disease occurs frequently. Unfortunately, highly effective agents with tolerable toxicity for this population remain absent.
Palliative chemotherapy is often provided to patients with symptomatic or rapidly progressing metastatic ACC patients, but generally yield few responses (2). Alterations in the MYB signaling pathway (65%) are considered to be a hallmark of ACC (3), which could upregulate several target genes, including vascular endothelial growth factor A (VEGFA), fibroblast growth factor 2 (FGF2), and c-KIT (4). Clinical trials have evaluated the efficacy of multi-targeted tyrosine kinase inhibitors (TKIs), including lenvatinib, sunitinib, sorafenib, axitinib, dasatinib and dovitinib in recurrent and metastatic ACC with response rates ranging from 0% to 15.6% (5-13).
Anlotinib is a novel TKI that selectively targeting VEGFR2/3, FGFR1-4, platelet-derived growth factor receptor (PDGFR), c-KIT and Ret (14). Anlotinib exhibits promising antitumor effect in a variety of malignancies, and was approved for the treatment of non-small cell lung cancer, small cell lung cancer, and soft tissue sarcomas in China (15-17). However, the value of anlotinib in metastatic ACC remains unclear. In the current study, we retrospectively assessed the efficacy and safety of anlotinib in patients with metastatic ACC. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2433/rc).
Methods
Patient population
We retrospectively reviewed the clinical data of patients diagnosed with histologically confirmed metastatic ACC of any primary site from September 2018 to October 2020 at Sun Yat-sen University Cancer Center (SYSUCC) in China and patients treated with anlotinib was included in the analysis. The inclusion criteria included pathologically confirmed metastatic ACC patients, who were unsuitable for radical treatment such as surgery, intervention therapy or radiotherapy, as well as adequate organ function. The exclusion criteria included diseases involving vital blood vessels according to radiological imaging, history of hemorrhagic disorders, and hypertension that can’t be controlled by hypotensive drugs. The following patient characteristics were collected: age, gender, histopathological pattern, perineural invasion, lymph node metastasis, primary site, tumor margin, local recurrence, metastatic site, prior lines of locoregional and systemic therapy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (No. B2021-224) and individual consent for this retrospective analysis was waived.
Treatment and evaluation
All patients received anlotinib at a starting dose of 12 mg once daily. Each cycle was defined as 2 weeks on and 1 week off treatment. The data of anlotinib therapy were reviewed as follows: date of initial anlotinib treatment, reasons for treatment discontinuation, dose and toxicity of anlotinib, time to progression, date of death if available. All patients provided informed consent for anlotinib treatment. Dose reduction (to 10 mg or 8 mg) was allowed if the patient showed intolerable or uncontrolled drug-related toxicity. Treatment warranted a temporary interruption, no more than 3 weeks, if the drug-related toxicity could not be completely relieved by dose reduction and symptomatic treatment.
Tumor response was evaluated by magnetic resonance imaging (MRI) or computerized tomography (CT) every 6 weeks for primary lesion and metastatic lesion. Best overall response was recorded and assessed according to the Response Evaluation Criteria In Solid Tumors (RECIST), version 1.1. Progressive disease (PD) was defined as an increase in the maximum unidimensional measurement of the lesions by more than 20% on MRI or CT within 4 cycles. The objective response rate (ORR) was defined as the combined proportion of complete response (CR) and partial response (PR). Disease control rate (DCR) was defined as the proportion of patients without PD on record. Drug-related adverse events (AEs) were classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. These data were extracted from electronic medical records and radiological imaging.
A patient who discontinues the study will move into the follow-up period. Progression-free survival (PFS) was identified as the time from the start of anlotinib administration until disease progression, death, or the last follow-up. The overall survival (OS) was defined as the time from initiation of anlotinib treatment to death for any cause or the last contact date for living patients. The cut-off date for the statistical analysis of clinical outcomes was June 30, 2021.
Statistical analysis
Frequencies, means, and standard deviations were used to describe the tendency and distribution of the different parameters, including ORR, DCR and AEs. PFS and OS was estimated according to the Kaplan-Meier method. All statistical analysis was performed using SPSS version 24.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism (version 8).
Results
Patients’ characteristics
From September 2018 to October 2020, 19 metastatic ACC patients were enrolled. All pathologic diagnoses were confirmed at SYSUCC. The demographics and clinical baseline characteristics are shown in Table 1. The median age was 48 (range, 23–76) years, with 9 males and 10 females. The primary anatomical locations of ACC were as follows: submandibular gland (n=7, 37%), parotid gland (n=3, 16%), paranasal sinus (n=2, 11%), palate (n=3, 16%), nasopharynx (n=1, 5%), togue (n=2, 11%), and trachea (n=1, 5%). In addition, 2 patients (11%) had lymph node metastasis, 10 patients had perineural invasion, and 7 patients (37%) had positive margin. Local recurrence occurred in 5 patients. Lung was the most common site of metastasis. Pulmonary metastases were observed in 16 cases (84%).
Table 1
| Characteristic | No. of patients [%] |
|---|---|
| Age, years | |
| Median | 48 |
| Range | 23–76 |
| Gender | |
| Male | 9 [47] |
| Female | 10 [53] |
| Perineural invasion | |
| Absent | 9 [47] |
| Present | 10 [53] |
| Lymph node metastasis | |
| Absent | 17 [90] |
| Present | 2 [10] |
| Margin | |
| Negative | 12 [63] |
| Positive | 7 [37] |
| Primary site of ACC | |
| Submandibular gland | 7 [37] |
| Parotid gland | 3 [16] |
| Paranasal sinus | 2 [11] |
| Palate | 3 [16] |
| Nasopharynx | 1 [5] |
| Tongue | 2 [11] |
| Others | 1 [5] |
| Local recurrence | |
| Yes | 5 [26] |
| No | 14 [74] |
| Metastatic site | |
| Lung only | 12 [63] |
| Lung and others | 4 [21] |
| No lung | 3 [16] |
| Treatment of primary tumor | |
| Surgery only | 12 [63] |
| Surgery/radiotherapy | 4 [21] |
| Surgery/radiotherapy + adjuvant chemotherapy | 2 [11] |
| No bulky surgery | 1 [5] |
| Prior lines of systemic therapy | |
| 0 | 12 [63] |
| 1 | 5 [26] |
| 2 | 2 [11] |
ACC, adenoid cystic carcinoma.
As for treatment of primary site, 1 patient (5%) with non-bulky disease received biopsy and the others (95%, n=18) received surgical treatment. Among the surgically treated patients, 12 patients (63%) received surgical resections alone, 4 patients (21%) received adjuvant radiotherapy and 2 patients (11%) underwent adjuvant chemoradiotherapy. 7 patients (37%) had received prior systemic therapies for metastatic disease, including 2 patients with apatinib, 2 patients with PD-1 antibody + chemotherapy, 2 patients with chemotherapy and 1 patient with EGFR antibody + chemotherapy. Chemotherapeutic agents prior to anlotinib treatment were paclitaxel, platinum, and fluorouracil. All patients have new or progressive lesion(s) on a radiologic study and/or new or worsening disease-related symptoms detected within 6 months before anlotinib treatment.
Treatment outcomes
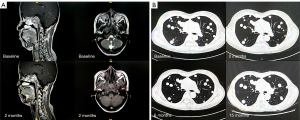
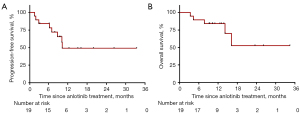
In all 19 patients, none of the patients achieved CR, 1 (5%) patient had PR, and 11 (58%) patients had SD as the best overall response. Additionally, 2 (11%) patients refused evaluation of treatment efficacy. The DCR for metastatic ACC was 63% (12/19) (Table 2). The only patient who achieved PR had local recurrence and bone metastasis. The pre- and post-treatment head MRs are shown in Figure 1A. By June 30, 2021, the median follow-up was 11.0 months. The median PFS was 10.1 (95% CI: 6.8–14.8) months, 10 (53%) disease progression events were observed; 3 patients were remaining on anlotinib treatment, and other patients discontinued from treatment for toxicity (n=2, 11%) or patients’ request (n=4, 21%). One patient with lung metastasis has a PFS more than 12 months (Figure 1B). The median OS was not reached. Three patients have been died due to tumor progression. The PFS and OS survival curves are shown in Figure 2.
Table 2
| Efficacy measure | No. of patients [%] |
|---|---|
| Best overall response | |
| Complete response | 0 |
| Partial response | 1 [5] |
| Stable disease | 11 [58] |
| Progression of disease | 5 [26] |
| Off before imaging | 2 [11] |
| Median progression-free survival, months (95% CI) | 15.2 (6.9–23.6) |
| Reasons for discontinuation | |
| Progression of disease | 10 [53] |
| Toxicity | 2 [11] |
| Patient choose | 4 [21] |
Safety and toxicity
The anlotinib-related toxicities are summarized in Table 3. The most common AEs included hypertension (n=6, 32%), oral pain (n=6, 32%), hypothyroidism (n=6, 32%), hand-foot skin syndrome (n=5, 26%), proteinuria (n=5, 26%), fatigue (n=4, 21%), and anorexia (n=4, 21%).
Table 3
| Toxicity | No. of patients [%] | Grade 1 | Grade 2 | Grade 3–4 |
|---|---|---|---|---|
| Hypertension | 6 [32] | 5 | 1 | 0 |
| Oral pain | 6 [32] | 5 | 0 | 1 |
| Hypothyroidism | 6 [32] | 6 | 0 | 0 |
| Hand foot syndrome | 5 [26] | 3 | 1 | 1 |
| Proteinuria | 5 [26] | 5 | 0 | 0 |
| Fatigue | 4 [21] | 4 | 0 | 0 |
| Anorexia | 4 [21] | 4 | 0 | 0 |
| Hemorrhage | 1 [5] | 0 | 1 | 0 |
| Fistula | 1 [5] | 0 | 1 | 0 |
ACC, adenoid cystic carcinoma.
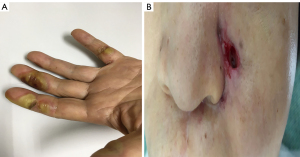
The grade 3 AEs were comprised of oral pain (n=1, 5.3%) and hand-foot skin syndrome (n=1, 5.3%), which were relieved by dose reduction of anlotinib to 10mg. Neither drug-related death nor grade 4 AEs occurred. However, two patients discontinued anlotinib treatment due to unacceptable hemorrhage and fistula, which were recovered after the drug withdrawal. The manifestations of typical AEs including hand foot syndrome and fistula are shown in Figure 3.
Discussion
To the best of our knowledge, this is the first study to demonstrate the efficacy and safety of anlotinib in metastatic ACC. Other TKIs have shown antitumor efficacy and tolerable toxicity in metastatic ACC (5-13). Accordingly, we conducted this study to evaluated this new TKI and try to make a comparison with other TKIs, aiming to provide a substituted treatment option for patients with metastatic ACC. In this study, the DCR of the metastatic ACC patients was 63.2% (12/19), the median PFS reached 10.1 months, and the overall safety was acceptable. The efficacy of anlotinib approximates to other TKIs in the treatment of relapsed or metastatic ACC according to published data (Table 4) (5).
Table 4
| Agent | Country | ACC | Study design | N | ORR (%) | DCR (%) | Median PFS or TTP | Progression criterion | References |
|---|---|---|---|---|---|---|---|---|---|
| Sunitinib | Canada | Recurrent/metastatic | Phase II, prospective | 14 | 0 (0/14) | 84.6 (11/13) | 7.2 (2.2–9.0) (TTP) | Radiographic | (6) |
| Sorafenib | Italy | Recurrent/metastatic | Phase II, prospective | 19 | 10.5 (2/19) | 47.4 (9/19) | 8.9 (not reported) (PFS) | Clinical | (8) |
| Sorafenib | United Kingdom | Recurrent/metastatic | Phase II, prospective | 19 | 10.5 (2/19) | 78.9 (15/19) | 11.3 (8.9–13.7) (PFS) | Clinical | (7) |
| Dovitinib | United States | Progressive | Phase II, prospective | 34 | 5.8 (2/34) | 64.7 (22/34) | 8.2 (7.3–11) (PFS) | Radiographic | (12) |
| Dovitinib | Republic of Korea | Metastatic/unresectable | Phase II, prospective | 32 | 3.1 (1/32) | 96.9 (31/32) | 6.0 (4.4–7.6) (PFS) | Radiographic | (11) |
| Axitinib | United States | Recurrent/metastatic | Phase II, prospective | 33 | 9.1 (3/33) | 75.8 (25/33) | 5.7 (5.3–9.1) (PFS) | Radiographic | (9) |
| Regorafenib | United States | Progressive, recurrent/metastatic | Phase II, prospective | 38 | 0 (0/38) | 44.7 (17/38) | – | Radiographic | (13) |
| Dasatinib | United States | Recurrent/metastatic | Phase II, prospective | 40 | 2.5 (1/40) | 52.5 (21/40) | 4.8 (1.8–6.9) (PFS) | Radiographic | (10) |
| Lenvatinib | United States | Recurrent/metastatic | Phase II, prospective | 32 | 15 (5/32) | 90.6 (29/32) | 17.5 (7.2–NR) (PFS) | Radiographic and/or symptomatic | (5) |
| Anlotinib | China | Metastatic | Retrospective | 19 | 5.3 (1/19) | 63.2 (12/19) | 10.1 (6.8–14.8) (PFS) | Radiographic | – |
ACC, adenoid cystic carcinoma; ORR, overall response rate; DCR, disease control rate; PFS, progressive free survival; TTP, time to progression; NR, not reach.
ACC is a slow growing but aggressive cancer mainly originating at secretory glands of head and neck, and occasionally at breast or other sites. ACC is generally incurable and has continuously decreasing survival rates over time (18). The survival rates reported varied among different institutions and countries. Previous retrospective studies showed that the 5- and 10-year OS rates of ACC were 76.8–90.3% and 58.0–79.9% (19-25). Prognostic factors for ACC include age, original site, tumor size, perineural or lymph vascular invasion, histological pattern, lymph node and distant metastasis (19). ACC can occur in all age groups but is commonly diagnosed between 50 to 60 years of age. Age is one of the main clinical prognostic factors determining event-free survival of patients with ACC (26). Patients in our study were younger with the median age of 47.9 years. ACC is also characterized by high risk of distant metastasis (27-29). Lung is the most common site of metastasis, accounting for 94.4% of all distant metastatic events (30). Patients with distant metastasis carry poor long-term survival outcomes, with a median survival of 5.8 years (28). The 10-year OS rates was only 55.2% for pulmonary metastasis (30). Thus, distant metastasis comes to be the most challenging events of ACC.
Management for ACC is difficult for several reasons. Surgery has been considered as the mainstay of ACC treatment (31). The standard treatment for resectable ACC is radical resection. However, residual disease after surgery is common owing to the complicated anatomic structures of salivary glands and perineural invasion of ACC. Postoperative radiotherapy is often considered as an important adjuvant treatment (32). Nevertheless, margin-positive or unresected cases could be insensitive to radiotherapy. Unfortunately, postoperative radiotherapy might only delay local recurrence, and bring no benefits to OS (33,34). In this study, most patients (94.7%) received surgical treatment, but only 31.6% of ACC patients received postoperative radiotherapy.
The systemic chemotherapy is employed for palliation of advanced ACC, and the regimens and agents evolve over time. The response rate to cytotoxic chemotherapy (2) was unsatisfactory. For metastatic ACC, cytotoxic chemotherapy may only improve the quality of life instead of survival outcomes (31,35). Thus, novel agents are urgently needed for advanced ACC. Immune checkpoint inhibitors have demonstrated antitumor activity in other malignancies of head and neck, but their impact in metastatic ACC seems to be limited due to the immune-excluded microenvironment of ACC (36-38). Researches focusing on the genomic landscape of ACC have identified several biomarkers including MYB, c-KIT, FGFR2, VEGFR, and Notch 1, which provided potential therapeutic targets for ACC (39-41). In a study including 30 Chinese ACC patients, 20.0% of patients harbored mutations sensitive to off-the-shelf targeted therapeutic drugs, supporting the clinical application of targeted therapies for patients with metastatic ACC (42). However, single-target agents, such as imatinib for c-KIT, lapatinib for EGFR, yielded unsatisfactory results in ACC (43,44). The efficacy and safety of several multi-targeted TKI agents in recurrent or metastatic ACC were evaluated in recent studies. In a phase II study including patients treated with lenvatinib, the ORR was 15.6% (5/32 confirmed PRs) and the median PFS was 17.5 months (5). Other similar drugs also showed certain antitumor activity in ACC, such as axitinib (ORR, 9.1%; median PFS, 5.7 months), sorafenib (ORR, 11%; median PFS, 8.9–11.3 months) and dovitinib (ORR, 3.1–6.0%; median PFS, 6.0–8.2 months) (7-9,11,12).
Anlotinib is a novel multi-targeted TKI and its target spectrum is similar to lenvatinib (including VEGFR, PDGFR, FGFR, and c-KIT), but their affinity with these targets is different (14). In the present study, the ORR of anlotinib in metastatic ACC patients was 5.3 (1/19). Up to 63.2% (12/19) achieved at least stable disease. The median PFS was 10.1 months, which is similar to other multi-targeted agents except lenvatinib. The efficacy of anlotinib in metastatic ACC is comparable with other multi-targeted TKI agents according to published studies (5-13). However, given the heterogeneity of the populations and methodology, comparison across studies is generally suboptimum. Thus, randomized controlled trials should be conducted to compare these agents.
Predictive biomarkers are expected to identify patients who are sensitive to TKI treatment, but no pragmatic predictive biomarker could be found to guide the use of TKI in ACC (5,9), even with high throughput methods such as genomic sequencing. Given the promising antitumor activity of anlotinib in ACC, effort should be made to develop predictive biomarkers to optimize the use of anlotinib. Serum CCL2 level is considered effective to predict the efficacy of anlotinib in advanced non-small cell lung cancer patients (45), and may be a potential biomarker to guide the use of anlotinib in ACC.
Hemorrhage is a common but often unacceptable AEs of anti-angiogenic drugs, which is generally caused by vascular erosion or just spontaneous tumor bleeding (46).Other mild or moderate side effects caused by TKIs, including dry mouth, sticky saliva, feeling ill, use of painkillers, and weight loss, might also deteriorate the quality of life (47). Thus, the side effect of multi-targeted TKI in metastatic ACC should be concerned. The AE profile of anlotinib in metastatic ACC is similar with anlotinib in other malignancies and other multi-targeted TKI agents (5,17). There was no grade 4 AEs in the present study, although two patients (10.5%) suffered grade 3 AEs, including oral pain and hand-foot skin syndrome. These two patients received a dose reduction of anlotinib to 10 mg. Also, 2 patients discontinued the treatment of anlotinib because of grade 2 hemorrhage and nasopharynx fistula. Both patients were recovered after withdraw of anlotinib. The overall toxicity of anlotinib was relatively well tolerated in metastatic ACC.
Our study has several limitations that should be brought up. First, as a retrospective study, the clinical data may not be completely reliable due to the recall bias, which weakens the evidence strength of this study. However, given the relatively low incidence of ACC, randomized controlled studies were difficult to conducted, especially for metastatic disease and the current study still provide some clinical evidence of anlotinib in metastatic ACCs. Second, the sample size of this study is small, which may decrease the statistical power and caused false negative result. Moreover, in view of the boarding targets of anlotinib, predictive biomarkers of anlotinib in metastatic ACC should be explored.
In conclusion, the present study demonstrates the anti-tumor activity and tolerable toxicity of anlotinib for metastatic ACC patients. Given the lack of highly effective agents for metastatic ACC patients, anlotinib could be considered as a potential treatment option. Notably, hemorrhage and fistula during anlotinib treatment should be concerned. Further investigations and elucidations are needed to carefully determine the value of anlotinib in metastatic ACC.
Acknowledgments
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 81973384 to Dr. Xiaopeng Tian), the Special Support Program of Sun Yat-sen University Cancer Center (No. PT19020401 to Dr. Qingqing Cai), the Science and Technology Planning Project of Guangzhou, China (No. 202002030205 to Dr. Qingqing Cai), and the Clinical Oncology Foundation of Chinese Society of Clinical Oncology (No. Y-XD2019-124 to Dr. Qingqing Cai).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2433/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2433/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2433/coif). XT reports grants from the National Natural Science Foundation of China (No. 81973384). QC reports grants from the Special Support Program of Sun Yat-sen University Cancer Center (No. PT19020401), the Science and Technology Planning Project of Guangzhou, China (No. 202002030205), and the Clinical Oncology Foundation of Chinese Society of Clinical Oncology (No. Y-XD2019-124). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (No. B2021-224) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg 1997;174:495-8. [Crossref] [PubMed]
- Laurie SA, Ho AL, Fury MG, et al. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol 2011;12:815-24. [Crossref] [PubMed]
- Ho AS, Kannan K, Roy DM, et al. The mutational landscape of adenoid cystic carcinoma. Nat Genet 2013;45:791-8. [Crossref] [PubMed]
- Persson M, Andrén Y, Mark J, et al. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A 2009;106:18740-4. [Crossref] [PubMed]
- Tchekmedyian V, Sherman EJ, Dunn L, et al. Phase II Study of Lenvatinib in Patients With Progressive, Recurrent or Metastatic Adenoid Cystic Carcinoma. J Clin Oncol 2019;37:1529-37. [Crossref] [PubMed]
- Chau NG, Hotte SJ, Chen EX, et al. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol 2012;23:1562-70. [Crossref] [PubMed]
- Thomson DJ, Silva P, Denton K, et al. Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck. Head Neck 2015;37:182-7. [Crossref] [PubMed]
- Locati LD, Perrone F, Cortelazzi B, et al. A phase II study of sorafenib in recurrent and/or metastatic salivary gland carcinomas: Translational analyses and clinical impact. Eur J Cancer 2016;69:158-65. [Crossref] [PubMed]
- Ho AL, Dunn L, Sherman EJ, et al. A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann Oncol 2016;27:1902-8. [Crossref] [PubMed]
- Wong SJ, Karrison T, Hayes DN, et al. Phase II trial of dasatinib for recurrent or metastatic c-KIT expressing adenoid cystic carcinoma and for nonadenoid cystic malignant salivary tumors. Ann Oncol 2016;27:318-23. [Crossref] [PubMed]
- Keam B, Kim SB, Shin SH, et al. Phase 2 study of dovitinib in patients with metastatic or unresectable adenoid cystic carcinoma. Cancer 2015;121:2612-7. [Crossref] [PubMed]
- Dillon PM, Petroni GR, Horton BJ, et al. A Phase II Study of Dovitinib in Patients with Recurrent or Metastatic Adenoid Cystic Carcinoma. Clin Cancer Res 2017;23:4138-45. [Crossref] [PubMed]
- Ho AL, Sherman EJ, Baxi SS, et al. Phase II study of regorafenib in progressive, recurrent/metastatic adenoid cystic carcinoma. J Clin Oncol 2016;34:6096. [Crossref]
- Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [Crossref] [PubMed]
- Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018;118:654-61. [Crossref] [PubMed]
- Cheng Y, Wang Q, Li K, et al. OA13.03 Anlotinib as third-line or further-line treatment in relapsed SCLC: a multicentre, randomized, double-blind phase 2 trial. J Thorac Oncol 2018;13:S351-2. [Crossref]
- Chi Y, Fang Z, Hong X, et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients with Refractory Metastatic Soft-Tissue Sarcoma. Clin Cancer Res 2018;24:5233-8. [Crossref] [PubMed]
- Lloyd S, Yu JB, Wilson LD, et al. Determinants and patterns of survival in adenoid cystic carcinoma of the head and neck, including an analysis of adjuvant radiation therapy. Am J Clin Oncol 2011;34:76-81. [Crossref] [PubMed]
- Ellington CL, Goodman M, Kono SA, et al. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973-2007 Surveillance, Epidemiology, and End Results data. Cancer 2012;118:4444-51. [Crossref] [PubMed]
- Shen W, Sakamoto N, Yang L. Model to Predict Cause-Specific Mortality in Patients with Head and Neck Adenoid Cystic Carcinoma: A Competing Risk Analysis. Ann Surg Oncol 2017;24:2129-36. [Crossref] [PubMed]
- Bjørndal K, Krogdahl A, Therkildsen MH, et al. Salivary gland carcinoma in Denmark 1990-2005: a national study of incidence, site and histology. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol 2011;47:677-82. [Crossref] [PubMed]
- Takebayashi S, Shinohara S, Tamaki H, et al. Adenoid cystic carcinoma of the head and neck: a retrospective multicenter study. Acta Otolaryngol 2018;138:73-9. [Crossref] [PubMed]
- Jeong IS, Roh JL, Cho KJ, et al. Risk factors for survival and distant metastasis in 125 patients with head and neck adenoid cystic carcinoma undergoing primary surgery. J Cancer Res Clin Oncol 2020;146:1343-50. [Crossref] [PubMed]
- He S, Li P, Zhong Q, et al. Clinicopathologic and prognostic factors in adenoid cystic carcinoma of head and neck minor salivary glands: A clinical analysis of 130 cases. Am J Otolaryngol 2017;38:157-62. [Crossref] [PubMed]
- Ciccolallo L, Licitra L, Cantú G, et al. Survival from salivary glands adenoid cystic carcinoma in European populations. Oral Oncol 2009;45:669-74. [Crossref] [PubMed]
- Atallah S, Casiraghi O, Fakhry N, et al. A prospective multicentre REFCOR study of 470 cases of head and neck Adenoid cystic carcinoma: epidemiology and prognostic factors. Eur J Cancer 2020;130:241-9. [Crossref] [PubMed]
- Kokemueller H, Eckardt A, Brachvogel P, et al. Adenoid cystic carcinoma of the head and neck--a 20 years experience. Int J Oral Maxillofac Surg 2004;33:25-31. [Crossref] [PubMed]
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck--An update. Oral Oncol 2015;51:652-61. [Crossref] [PubMed]
- Yu T, Gao QH, Wang XY, et al. Malignant sublingual gland tumors: a retrospective clinicopathologic study of 28 cases. Oncology 2007;72:39-44. [Crossref] [PubMed]
- Seok J, Lee DY, Kim WS, et al. Lung metastasis in adenoid cystic carcinoma of the head and neck. Head Neck 2019;41:3976-83. [Crossref] [PubMed]
- Iseli TA, Karnell LH, Graham SM, et al. Role of radiotherapy in adenoid cystic carcinoma of the head and neck. J Laryngol Otol 2009;123:1137-44. [Crossref] [PubMed]
- Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck 2004;26:154-62. [Crossref] [PubMed]
- Chen Y, Zheng ZQ, Chen FP, et al. Role of Postoperative Radiotherapy in Nonmetastatic Head and Neck Adenoid Cystic Carcinoma. J Natl Compr Canc Netw 2020;18:1476-84. [Crossref] [PubMed]
- Katz TS, Mendenhall WM, Morris CG, et al. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck 2002;24:821-9. [Crossref] [PubMed]
- Adelstein DJ, Koyfman SA, El-Naggar AK, et al. Biology and management of salivary gland cancers. Semin Radiat Oncol 2012;22:245-53. [Crossref] [PubMed]
- Linxweiler M, Kuo F, Katabi N, et al. The Immune Microenvironment and Neoantigen Landscape of Aggressive Salivary Gland Carcinomas Differ by Subtype. Clin Cancer Res 2020;26:2859-70. [Crossref] [PubMed]
- Wang F, Xie X, Song M, et al. Tumor immune microenvironment and mutational analysis of tracheal adenoid cystic carcinoma. Ann Transl Med 2020;8:750. [Crossref] [PubMed]
- Wolkow N, Jakobiec FA, Afrogheh AH, et al. PD-L1 and PD-L2 Expression Levels Are Low in Primary and Secondary Adenoid Cystic Carcinomas of the Orbit: Therapeutic Implications. Ophthalmic Plast Reconstr Surg 2020;36:444-50. [Crossref] [PubMed]
- Sajed DP, Faquin WC, Carey C, et al. Diffuse Staining for Activated NOTCH1 Correlates With NOTCH1 Mutation Status and Is Associated With Worse Outcome in Adenoid Cystic Carcinoma. Am J Surg Pathol 2017;41:1473-82. [Crossref] [PubMed]
- Mitani Y, Li J, Rao PH, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin Cancer Res 2010;16:4722-31. [Crossref] [PubMed]
- Stephens PJ, Davies HR, Mitani Y, et al. Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest 2013;123:2965-8. [Crossref] [PubMed]
- Wang S, Yu Y, Fang Y, et al. Whole-exome sequencing reveals genetic underpinnings of salivary adenoid cystic carcinoma in the Chinese population. J Genet Genomics 2020;47:397-401. [Crossref] [PubMed]
- Guigay J, Bidault F, Temam S, et al. Antitumor activity of imatinib in progressive, highly expressing KIT adenoid cystic carcinoma of the salivary glands: A phase II study. J Clin Oncol 2007;25:6086. [Crossref]
- Agulnik M, Cohen EW, Cohen RB, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol 2007;25:3978-84. [Crossref] [PubMed]
- Lu J, Zhong H, Chu T, Zhang X, Li R, Sun J, et al. Role of anlotinib-induced CCL2 decrease in anti-angiogenesis and response prediction for nonsmall cell lung cancer therapy. Eur Respir J. 2019;53: [Crossref] [PubMed]
- Chou CT, Rath TJ, Johnson JT, et al. Catastrophic Hemorrhage After Chemoradiation for Advanced Stage Oropharyngeal Carcinoma: A Case Series. Laryngoscope 2021;131:1049-52. [Crossref] [PubMed]
- Locati LD, Galbiati D, Calareso G, et al. Patients with adenoid cystic carcinomas of the salivary glands treated with lenvatinib: Activity and quality of life. Cancer 2020;126:1888-94. [Crossref] [PubMed]


