
Pneumocystis jirovecii pneumonia in non-Hodgkin’s lymphoma after rituximab-based chemotherapy: a case series
Introduction
Pneumocystis jirovecii pneumonia (PCP) is a common disease occurring in patients with acquired immunodeficiency syndrome (AIDS). As immunosuppressive medications become more widely used, the incidence of PCP has been increasing in patients without human immunodeficiency virus (HIV) infection due to the use of glucocorticoid therapy and immunosuppressive medications, solid organ transplantation, allogeneic hematopoietic stem cell transplantation, and congenital immunodeficiency syndromes (1).
Recently, several studies have reported that there is an increased risk of PCP in patients with non-Hodgkin’s lymphoma (NHL) treated with rituximab-containing regimens (2-4). Lee’s study estimated the incidence of and risk factors for PCP infection in diffuse large B-cell lymphoma (DLBCL) and showed the importance of PCP prophylaxis during R-CHOP treatment (2). Without PCP prophylaxis, the approximate incidence of PCP may be up to 15% in such patients (2,5). And the risk of PCP increased in patients received R-CHOP-14 (3). More importantly, the mortality of PCP can reach 29% in patients with hematologic malignancies (6), which is significantly higher than that of those with HIV (6–7%) (7,8). Only isolated cases of PCP in NHL have been reported in the literature, and information on clinical presentation, criteria for clinical diagnosis, and prognosis remains unclear.
The objective of this study was to review the clinical findings and outcomes of the instances of PCP in patients with NHL seen at our institution, in the hope of providing some guidance for the prevention and treatment of these patients. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1216/rc).
Methods
This was a single-center and retrospective cohort study of NHL patients diagnosed at the First Affiliated Hospital of Wenzhou Medical University between 30 June 2014 and 1 June 2020, who developed PCP. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board at the First Affiliated Hospital of Wenzhou Medical University (No. KY2022-R066). Individual consent for this retrospective analysis was waived. Patients who displayed clinical symptoms such as fever, cough, sputum, or dyspnea, had radiological features including patch bilateral ground-glass opacity on chest radiography or chest computed tomography (CT), and received microbiological confirmation were classified as ‘definite PCP’. Patients who had clinical and radiological features strongly suggestive of PCP but without microbiological examination or had a negative test result were classified as ‘probable PCP’. A positive microbiological test was defined as clinical findings of pneumonia with identification of Pjiroveci organisms by smear, polymerase chain reaction (PCR), or next-generation sequencing (NGS) in sputum, bronchoalveolar lavage (BAL) fluid, or lung biopsy specimens. Their medical records were reviewed and the following data were abstracted: age at NHL diagnosis, gender, date at diagnosis of PCP, symptoms at PCP diagnosis, laboratory and radiographic findings at the time of PCP diagnosis, corticosteroid dose, any other immunosuppressive treatment or comorbidities, treatment of PCP, and outcome. Participants were followed up until death or 31 August 2021, whichever came first. As information bias may occur in description of clinical symptoms, the diagnosis of PCP should be comprehensively assessed by clinical symptoms, signs, and laboratory examination results. We recruited all consecutive patients with clinical and radiographic findings, so the number of cases in our center during the study period determined the sample size.
Statistical analysis
The data were analyzed using descriptive statistics and paired-samples t-test. A P value <0.05 was considered statistically significant. Statistical analyses were conducted with the software SPSS 21.0 (IBM Corp., Armonk, NY, USA).
Results
During the study period, we included a total of 15 patients who acquired PCP during the course of chemotherapy (Table 1). Of these 15, 7 were classified as ‘definite PCP’; the other 8 were classified as ‘probable PCP’. All patients were treated with chemotherapeutic regimens including rituximab such as R-CHOP (rituximab 375 mg/m2 d0, cyclophosphamide 750 mg/m2 d1, hydroxydaunorubicin 50 mg/m2 d1, vincristine 1.4 mg/m2 d1, prednisone 100 mg d1–5), R-DHAP (rituximab 375 mg/m2 d0, cis-platinum 1,000 mg/m2 d1, cytarabine 2 g/m2 q12h d2, dexamethasone 40 mg d1–4), and R-CVP (rituximab 375 mg/m2 d0, cyclophosphamide 750 mg/m2 d1, vincristine 1.4 mg/m2 d1, prednisone 100 mg d1–5). Patients did not receive PCP prophylaxis during chemotherapy. All participants received combined therapy including high-dose trimethoprim/sulfamethoxazole (TMP/SMZ), caspofungin, steroids, and empiric broad-spectrum antibiotics (as bacterial infection could not be excluded initially). Ventilatory support was required by 3 participants, so they were admitted to the intensive care unit (ICU), and only 1 patient died from PCP.
Table 1
| Patient/gender | Age at PCP diagnosis (years) | Symptoms | Lymphoma type/stage | Number of cycles before PCP infection | Microbiological testing | ICU admission | Total lymphocyte count pretreatment [ref. range: (1,500–4,000)×106/mL] | Total lymphocyte count at time of PCP diagnosis | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1/M | 49 | Fever, cough, sputum, and dyspnea | DLBCL/IVA | 3 | Sputum culturing positive | N | 1,590 | 1,010 | Survived PCP, still alive |
| 2/F | 68 | Fever, cough, sputum, and dyspnea | DLBCL/IIA | 4 | Negative | N | 2,740 | 790 | Survived PCP, still alive |
| 3/M | 75 | Fever, cough, sputum, and dyspnea | DLBCL/IIA | 3 | Negative | N | 810 | 400 | Survived PCP, still alive |
| 4/M | 36 | Fever and cough | DLBCL/IIA | 6 | Negative | N | 1,730 | 380 | Survived PCP, still alive |
| 5/M | 30 | Fever and dyspnea | DLBCL/IIIA | 4 | Negative | N | 1,350 | 750 | Survived PCP, still alive |
| 6/M | 71 | Fever and dyspnea | DLBCL/IIA | 4 | Sputum NGS positive | N | 2,280 | 270 | Survived PCP, still alive |
| 7/M | 55 | Fever and dyspnea | DLBCL/IIIA | 5 | BAL NGS positive | N | 1,850 | 520 | Survived PCP, died from other disease |
| 8/M | 56 | Fever and dyspnea | DLBCL/IIA | 3 | Negative | Y (7 days) | 3,070 | 1,730 | Survived PCP, still alive |
| 9/M | 66 | Fever and dyspnea | DLBCL/IIA | 2 | Negative | N | 2,450 | 660 | Survived PCP, died from other disease |
| 10/F | 70 | Fever | DLBCL/IIIA | 4 | BAL NGS positive | N | 2,420 | 190 | Survived PCP, still alive |
| 11/F | 59 | Fever | DLBCL/IIA | 5 | Negative | Y (7 days) | 1,290 | 1,210 | Survived PCP, still alive |
| 12/M | 50 | Fever and dyspnea | FL/IVA | 5 | Sputum culturing positive | N | 1,910 | 200 | Died from PCP |
| 13/M | 56 | Fever, cough, and dyspnea | FL/IIIA | 3 | negative | N | 1,570 | 480 | Survived PCP, still alive |
| 14/M | 67 | Fever, cough, sputum, and dyspnea | FL/IIIA | 2 | BAL NGS and sputum culturing positive | Y(17 days) | 1,900 | 340 | Survived PCP, still alive |
| 15/M | 61 | Fever, cough, sputum, and dyspnea | MZL/IIA | 4 | BAL NGS positive | N | 1,490 | 490 | Survived PCP, still alive |
DLBCL, diffuse large B cell lymphoma; PCP, Pneumocystis jirovecii pneumonia; FL, follicular cell lymphoma; MZL, marginal zone lymphoma; ICU, intensive care unit; BAL, bronchoalveolar lavage; NGS, next-generation sequencing.
The age of patients with PCP ranged from 30 to 75 years, and PCP was complicated by chemotherapy after about 4 courses (range, 2 to 6 courses). Most patients had a standard lymphocyte count pretreatment, and 14 of 15 patients (93.3%) had lymphopenia at the time of PCP infection, 6 of whom had absolute lymphocyte count ≤0.5×109/L. All participants underwent etiology inspection tests during the course of PCP. A total of 7 patients had a positive result of microbiological testing: sputum culture was positive in 3 patients, NGS was positive in 3 patients, and 1 was positive result in both sputum culture and NGS. A total of 14 patients survived PCP, 2 of whom eventually died from other diseases; 12 patients are currently alive.
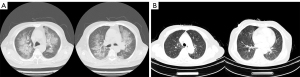
All patients had fever before the diagnosis of PCP, and dyspnea was the second most common symptom. Laboratory and radiographic findings in patients with PCP are displayed in Table 2 and Figure 1. Both CRP and LDH were routinely measured in follow-up examinations, and we recorded laboratory test results 7–10 days before diagnosis. In most cases, CRP and LDH had increased to varying degrees before the onset of clinical symptoms (Figure 1), and the difference was statistically significant (P=0.001, P=0.002, respectively). All patients had an abnormal elevation of CRP, the median was 79.3 mg/L (range: 37.8 to 105; reference range: 0 to 8). The median serum LDH of all participants was 535 U/L (range: 235 to 841; reference range: 0 to 247). The (1,3)-β-D-glucan test was shown to be another sensitive index for PCP. Abnormal results were observed in 13 of 15 patients in the (1,3)-β-D-glucan test, and the median was 224.7 mg/mL (range: 67.4 to 1,893.9; reference range: 0 to 100.5). Serum aspergillus galactomannan (GM) antigen was negative in all patients. A total of 14 patients had a typical finding in CT scan such as bilateral ground-glass opacity (Figure 2), and 5 of 15 patients had pleural effusion at the time of diagnosis.
Table 2
| Variable | Pre-treatment/7–10 days before diagnosis | At diagnosis | P value |
|---|---|---|---|
| Laboratory findings | |||
| Total lymphocyte count, median (range)/mL | 1,850 (810–3,070) | 490 (190–1,730) | 0.001 |
| CRP, median (range) mg/L | 24.8 (3.02–74.6) | 79.3 (37.8–105) | 0.001 |
| Serum LDH, median (range) U/L | 320 (179–683) | 535 (235–841) | 0.002 |
| (1,3)-β-D-glucan test, median (range) mg/mL | – | 224.7 (67.4–1,893.9) | – |
| Microbiological test | |||
| Sputum culturing, n (%) | – | 3 (20.0) | – |
| NGS, n (%) | – | 5 (33.3) | – |
| Imaging findings | |||
| Bilateral ground-glass opacity, n (%) | – | 14 (93.3) | – |
| Cavitary mass, n (%) | – | 1 (6.7) | – |
| Pleural effusion, n (%) | – | 5 (33.3) | – |
NHL, non-Hodgkin’s lymphoma; PCP, Pneumocystis jirovecii pneumonia; CRP, C-reactive protein; NGS, next-generation sequencing; LDH, lactate dehydrogenase.

Discussion
We have reported a case series of patients with PCP occurring in the context of treatment for NHL. The clinical features of this group of patients appear similar to those of other PCP patients without AIDS (6,7), although the mortality rate in our cohort was lower than that reported in the existing literature (7).
Overall, PCP appears to be a rare infection in patients without AIDS. We identified only 15 cases of PCP despite the large volume of patients with NHL treated at our hospital. Reports of PCP in patients with NHL are scare in the literature and limited to isolated case reports (9-11). During the treatment of NHL, patients may occasionally develop PCP, but there have been no studies demonstrating benefit of PCP chemoprophylaxis for those patients (12). Also, there is no consensus on PCP prevention in the National Comprehensive Cancer Network (NCCN) guidelines.
Biotargeted therapies such as rituximab and corticosteroid treatment in chemotherapy are risks factors for PCP in non-HIV patients (13,14). In a study evaluating PCP in patients without AIDS, 90.5% of the patients had received systemic corticosteroids in the month prior to diagnosis (15). Compared with conventional chemotherapy, rituximab-based chemotherapy may increase the probability of PCP in patients with lymphoma (16,17).
At the early stage of PCP, chest radiography may appear normal in 5–10% patients (18,19). The pulmonary imaging changes on CT scan may be atypical infiltration, mostly located in the interstitium of the lung, and mainly manifested as lung texture thickening or reticular changes around the hilum of the lungs (20), shown as interstitial pneumonitis, alveolar-interstitial pneumonia, pulmonary fibrosis, and bronchiolitis obliterans organizing pneumonia (BOOP) (21), which is difficult to distinguish from rituximab-associated lung injury and viral pneumonia. In our study, 12 of 15 (80%) patients acquired PCP after 3–5 courses of treatment and all patients were excepted for the common viral pneumonia. According to our clinical experience, rituximab-associated lung injury usually occurs after 3 courses of biotargeted therapy (21), which is close to the time of PCP occurrence in most patients. The main clinical symptoms of PCP at the early stage were dry cough and fever, followed by chest pain, cyanosis, and dyspnea (1). However, it remains difficult to identify PCP patients at the early stage due to the atypical signs and symptoms. The gold standard for clinical diagnosis of PCP is etiology examination (20), but the positive rate of conventional sputum smear remains low. The LDH is an important assessment for lymphoma after treatment, which may rise when lymphoma progresses. It has been shown that a high level of LDH is associated with severe PCP and higher 90-day mortality (13). We found that the rise of LDH can occur before all of the clinical symptoms such as fever and cough. In the case of lymphoma monitoring, LDH may be a sensitive index for the early diagnosis of PCP. In our cohort, 12 of 15 cases had a rise of LDH during clinical follow-up and began to exhibit clinical symptoms such as fever and cough after several days, and were finally diagnosed as PCP. Both CRP and (1,3)-β-D-glucan testing are routine monitoring assessments in patients with NHL (13,22), which can be positive in the early stage of PCP, even in the presence of clinical symptoms. In case 4 and case 7, we monitored CRP, LDH, arterial blood gas analysis, and CT scan during follow-up. We found that hypoxemia appeared earlier than radiographic imaging features. So, we have identified that blood gas analysis should be performed promptly when the rise of LDH and CRP are monitored as abnormal elevations in patients with NHL as it was a reliable and sensitive index for ‘probable PCP’.
Patients who are eligible to receive bronchoscopy and NGS for BAL are encouraged to undergo those examinations at the early stage. In case 7, we found a total of 129 reads mapped to Pneumocystis jirovecii in the reference database. The coverage of referenced Pneumocystis jirovecii genome was 1% (Figure 3). In solid organ transplantation, antimicrobial prophylaxis has been found to be a successful method in preventing PCP in patients with immunosuppression, while the standard prevention program in NHL remains unclear (23). Early prevention and treatment are important measures for patients with PCP to achieve prolonged survival.
In our study, 2 of the 15 patients needed ventilator-assisted ventilation treatment, and 14 patients experienced improved hypothermia and dyspnea after treatment and their lung performance was significantly improved on the CT scans. In all, 7 of 15 patients received a positive result from microbiological testing, 5 of whom had nucleic acid sequences indicating infection of Pneumocystis carinii through NGS. Studies have found that the mortality rate of PCP in non-HIV-infected patients is higher than that of HIV-infected patients (6-8). In addition, the in-hospital mortality rate of PCP in patients with autoimmune diseases is as high as 46% (24). However, there are few reports on the prognosis of NHL patients with PCP after treatment. The effectiveness of TMP/SMZ for PCP is limited with adverse events. Studies have shown that combination therapy with caspofungin and TMP/SMZ is an effective and promising, as the associated adverse events are comparable to those of TMP/SMZ monotherapy (25,26). Only 1 patient died from PCP, the mortality in our cohort was 6.7%, which is significantly lower than that reported in previous literature. In our report, patients received both TMP/SMZ and caspofungin as the therapy for PCP. We assume that early diagnosis and combination therapy with caspofungin and TMP/SMZ of PCP can significantly improve the prognosis of patients.
This study had the following disadvantages. Firstly, it was a retrospective study. Although 3/5 of the participants were suspected to be diagnosed with PCP, with typical lung changes and good treatment effect, no clear etiological basis was found. Secondly, most patients in our study received empiric broad-spectrum antibiotics treatment, although we could not confirm whether the patient was complicated with other fungal, bacterial, and viral infections. Thirdly, this was a single-center and retrospective study, thus selection bias was difficult to avoid.
In summary, this study demonstrates that dynamic monitoring of CRP, LDH and (1,3)-β-D-glucan test in patients with NHL is very significant for the early detection and diagnosis of PCP. Additionally, NGS is a rapid and sensitive method for the diagnosis of PCP. Bronchoscopy and NGS for BAL are extremely significant for diagnosis of PCP. When patients are classified as ‘probable PCP’, early and effective treatment has obvious significance to improve their prognosis.
Acknowledgments
Funding: The authors are thankful for the financial support from the Natural Science Foundation of Zhejiang Province (LY20H080004) and Major R&D Project of Wenzhou Science and Technology Bureau (ZY2021013).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1216/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1216/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1216/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board at the First Affiliated Hospital of Wenzhou Medical University (No. KY2022-R066). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Truong J, Ashurst JV. Pneumocystis Jirovecii Pneumonia. 2022 Feb 17. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing, 2022.
- Lee JY, Kang M, Suh KJ, et al. Pneumocystis jirovecii pneumonia in diffuse large B-cell Lymphoma treated with R-CHOP. Mycoses 2021;64:60-5. [Crossref] [PubMed]
- Tadmor T, McLaughlin P, Polliack A. A resurgence of Pneumocystis in aggressive lymphoma treated with R-CHOP-14: the price of a dose-dense regimen? Leuk Lymphoma 2010;51:737-8. [Crossref] [PubMed]
- Hardak E, Oren I, Dann EJ, et al. The increased risk for pneumocystis pneumonia in patients receiving rituximab-CHOP-14 can be prevented by the administration of trimethoprim/sulfamethoxazole: a single-center experience. Acta Haematol 2012;127:110-4. [Crossref] [PubMed]
- Kamel S, O'Connor S, Lee N, et al. High incidence of Pneumocystis jirovecii pneumonia in patients receiving biweekly rituximab and cyclophosphamide, adriamycin, vincristine, and prednisone. Leuk Lymphoma 2010;51:797-801. [Crossref] [PubMed]
- Pagano L, Fianchi L, Mele L, et al. Pneumocystis carinii pneumonia in patients with malignant haematological diseases: 10 years' experience of infection in GIMEMA centres. Br J Haematol 2002;117:379-86. [Crossref] [PubMed]
- Mansharamani NG, Garland R, Delaney D, et al. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest 2000;118:704-11. [Crossref] [PubMed]
- Sepkowitz KA, Brown AE, Telzak EE, et al. Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA 1992;267:832-7. [Crossref] [PubMed]
- Fukutani Y, Chigusa Y, Kondoh E, et al. Pneumocystis Pneumonia in Non-HIV Pregnant Women Receiving Chemotherapy for Malignant Lymphoma: Two Case Reports. Case Rep Obstet Gynecol 2017;2017:1073146. [Crossref] [PubMed]
- Chang H, Shih LY, Wang CW, et al. Granulomatous Pneumocystis jiroveci pneumonia in a patient with diffuse large B-cell lymphoma: case report and review of the literature. Acta Haematol 2010;123:30-3. [Crossref] [PubMed]
- Kim HS, Shin KE, Lee JH. Single nodular opacity of granulomatous pneumocystis jirovecii pneumonia in an asymptomatic lymphoma patient. Korean J Radiol 2015;16:440-3. [Crossref] [PubMed]
- Classen AY, Henze L, von Lilienfeld-Toal M, et al. Primary prophylaxis of bacterial infections and Pneumocystis jirovecii pneumonia in patients with hematologic malignancies and solid tumors: 2020 updated guidelines of the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology (AGIHO/DGHO). Ann Hematol 2021;100:1603-20. [Crossref] [PubMed]
- Gaborit BJ, Tessoulin B, Lavergne RA, et al. Outcome and prognostic factors of Pneumocystis jirovecii pneumonia in immunocompromised adults: a prospective observational study. Ann Intensive Care 2019;9:131. [Crossref] [PubMed]
- Juárez-Salcedo LM, Dalia S. Indolent non-Hodgkin lymphoma treatment in the new drugs era. Chin Clin Oncol 2020;9:81. [Crossref] [PubMed]
- Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc 1996;71:5-13. [Crossref] [PubMed]
- Kim YH, Kim JY, Kim DH, et al. Pneumocystis pneumonia occurrence and prophylaxis duration in kidney transplant recipients according to perioperative treatment with rituximab. BMC Nephrol 2020;21:93. [Crossref] [PubMed]
- Wei KC, Sy C, Wu SY, et al. Pneumocystis jirovecii pneumonia in HIV-uninfected, rituximab treated non-Hodgkin lymphoma patients. Sci Rep 2018;8:8321. [Crossref] [PubMed]
- Vogel MN, Vatlach M, Weissgerber P, et al. HRCT-features of Pneumocystis jiroveci pneumonia and their evolution before and after treatment in non-HIV immunocompromised patients. Eur J Radiol 2012;81:1315-20. [Crossref] [PubMed]
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014;44:1350-63. [Crossref] [PubMed]
- White PL, Backx M, Barnes RA. Diagnosis and management of Pneumocystis jirovecii infection. Expert Rev Anti Infect Ther 2017;15:435-47. [Crossref] [PubMed]
- Lands LC. New therapies, new concerns: rituximab-associated lung injury. Pediatr Nephrol 2010;25:1001-3. [Crossref] [PubMed]
- Damiani C, Le Gal S, Da Costa C, et al. Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1->3)-β-D-glucan for differential diagnosis of pneumocystis pneumonia and Pneumocystis colonization. J Clin Microbiol 2013;51:3380-8. [Crossref] [PubMed]
- Prasad GVR, Beckley J, Mathur M, et al. Safety and efficacy of prophylaxis for Pneumocystis jirovecii pneumonia involving trimethoprim-sulfamethoxazole dose reduction in kidney transplantation. BMC Infect Dis 2019;19:311. [Crossref] [PubMed]
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999;42:780-9. [Crossref] [PubMed]
- Tian Q, Si J, Jiang F, et al. Caspofungin combined with TMP/SMZ as a first-line therapy for moderate-to-severe PCP in patients with human immunodeficiency virus infection. HIV Med 2021;22:307-13. [Crossref] [PubMed]
- Wang ZG, Liu XM, Wang Q, et al. A retrospective study of patients with systemic lupus erythematosus combined with Pneumocystis jiroveci pneumonia treated with caspofungin and trimethoprim/sulfamethoxazole. Medicine (Baltimore) 2019;98:e15997. [Crossref] [PubMed]
(English Language Editor: J. Jones)



