
Imaging features of primary mucinous adenocarcinoma of bladder outlet and urethra: a case report and literature review
Introduction
Bladder cancer is one of the most common cancers and causes of death worldwide (1). Older people and men are more susceptible to from bladder cancer than any other group. With the growing number of elderly people and population growth, cancer-related deaths have increased markedly, partly due to a recurrence rate of up to 75% (1-3). Urothelial carcinoma, also known as transitional cell carcinoma, has an incidence of about 90–95%, which is higher than any other cancer. The further subdivision of non-urothelial histology consists of epithelial and non-epithelial. Small cell tumors, squamous cell carcinoma, and adenocarcinoma originate from epithelial cells. Squamous cell carcinoma and adenocarcinoma account for only 3% and 2% of primary bladder cancer cases, respectively (4). Previously published studies have found that primary bladder adenocarcinoma (PBA) is not a common tumor, accounting for approximately only 0.5–2% of all primary bladder malignancies (5,6). Elderly people, especially elderly men, are more likely to develop bladder cancer (7,8). Adenocarcinoma is most commonly seen in people aged 50–60 years (9). Other previously published studies have found that most patients diagnosed with bladder cancer are men in the 60–70-year age group (10,11). Urothelial carcinoma is the most common pathologic type of bladder cancer, accounting for 90% of cases, and is often found in the bladder trigone and lateral wall (12,13). Primary mucinous adenocarcinoma of the bladder, a subtype of PBA, are extremely uncommon, accounting for 20% of PBA (14,15). The pathogenesis of primary mucinous adenocarcinoma of the bladder is the progressive change from mucinous metaplasia and mucinous adenoma to mucinous adenocarcinoma (16). Diagnostic tests include urine biopsy, cystoscopy and cytology, and histopathological evaluation (17,18). Compared with urothelial carcinoma, studies on the pathogenesis and clinical process, treatment, and prognosis of primary mucinous adenocarcinoma of the bladder are lacking (19). Therefore, here, we present a case with primary mucinous adenocarcinoma of the bladder. The clinical manifestation of this case is atypical, in addition, the tumor is located at the bladder outlet and urethra. However, thanks to the timely detection of imaging examination, we have improved the symptoms and quality of life of patient through surgery and chemotherapy. Through follow-up, we know that the patient has survived so far. Through this case, we aim to suggest that when encountering patients with similar clinical symptoms and imaging manifestations, radiologists or clinicians can consider this disease in their differential diagnosis. In addition, after the case is confirmed, we consider that our treatment method can give clinicians more treatment ideas. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1547/rc).
Case presentation
A 62-year-old female was referred to an outpatient urology surgery service due to a 1-year history of narrowing of the urinary route and difficulties in urination. She had no symptoms, such as urinary pain, urgency, or hematuria. No significant abnormalities were found in other surgical examinations.
The patient first was examined by ultrasonography in the outpatient department. Ultrasonography revealed a hypoechoic mass of 6.5 cm × 3.8 cm × 4.4 cm at the bladder outlet and urethra. The mass had unclear boundaries, was irregular in shape, and heterogeneous in echo, with a punctate and irregular strong echo (Figure 1A). Color Doppler flow imaging (CDFI) showed a little blood flow signal in the tumor (Figure 1B). The residual urine volume of the bladder was about 283.3 mL. Later, the patient was admitted to the urology surgery, soon the patient underwent computed tomography (CT) examination. CT plain scan and enhancing scan displayed that the wall of bladder was thickened, and soft tissue density was seen at the bladder outlet and urethra. The mass demonstrated heterogeneous density and high-density punctate shadows on CT plain scan. The size of the mass was about 5.6 cm × 4.4 cm, with slight enhancement and an unclear partial boundary. The wall of the urethra was obviously thickened (Figure 2). The patient was initially diagnosed with a mass at the bladder outlet and urethra. Afterwards she underwent laparoscopic radical cystectomy, urethral resection, and ureterostomy under general anesthesia. Intraoperative findings revealed a hard and enlarged bladder outlet and urethra, and after incision, there was an ulcerative mass on the bladder posterior wall 2 cm away from the urethral stump. Postoperative pathological diagnosis of the patient was as follows: different cell structure arranged in solid clumps or small cords, with the formation of glandular lumen; lump tissue accompanied with neuroendocrine differentiation; some cells were signet ring cells; and focal calcification (Figure 3). Immunohistochemistry (IHC) outcomes were as follows: cytokeratin (CK) pan (positive), CK20 (positive), GATA binding protein 3 (GATA-3) (negative), synaptophysin (Syn) (positive), cluster of differentiation 56 (CD56) (partial positive), chromogranin A (CgA) (slightly positive), caudal type transcription factor-2 (CDX2) (negative), and Ki-67 proliferation nuclear antigen (Ki-67) (positive) (Figure 4). The diagnosis was mucinous adenocarcinoma (bladder outlet + urethra) and poorly differentiated adenocarcinoma (about 40%). In order to differentiate from metastatic lesions of intestinal origin, the patient underwent tumor markers examination and enteroscopy. The detection outcome of blood biochemical indicators indicated that carbohydrate antigen 72-4 (CA72-4) was abnormally increased, and carcinoembryonic antigen (CEA) was <0.5 ng/mL (Table 1). Enteroscope results were normal. The operation was successful, and 2 cycles of FOLFOX (leucovorin calcium + 5-fluorouracil + oxaliplatin)-6 regimen were performed. Furthermore, emission computed tomography (ECT) showed multiple bone metastases, zoledronic acid was applied to treat the bone metastases. The gastrointestinal reaction was grade 1, without bone marrow suppression or liver and kidney injury. The patient was stable and survived. Figure 5 is a brief description of the diagnosis and treatment of this case.
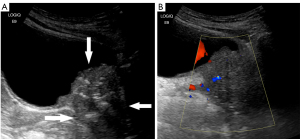
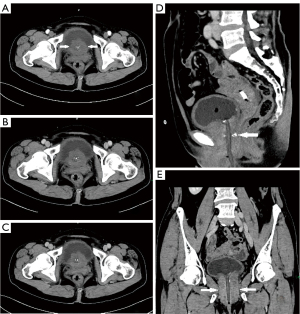
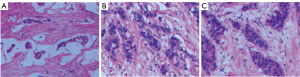

Table 1
| Item | Detection result (reference range) |
|---|---|
| CA72-4 (U/mL) | 35.11 (0–6.9) |
| CEA (ng/mL) | <0.05 (0–10) |
| CA199 (U/mL) | 9.7 (0–37) |
| CA125 (U/mL) | 4.28 (0–30.2) |
CA72-4, carbohydrate antigen 72-4; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 199; CA125, carbohydrate antigen 125.
All procedures performed in this study were in accordance with the ethical standards of the national research committee and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Primary mucinous adenocarcinoma of the bladder, including metastatic adenocarcinoma, primary mucinous adenocarcinoma of the urinary bladder, and urachal carcinoma, accounts for only 2% of primary bladder cancers. Primary mucinous adenocarcinoma of the bladder is a relatively uncommon primary bladder cancer (20). Previously published studies have found that a progressive change in the pathogenesis of primary mucinous adenocarcinoma of the bladder is from mucinous metaplasia and mucinous adenoma to mucinous adenocarcinoma (21). Patients who suffer from urachal remnants and bladder exstrophy have a higher risk of developing bladder adenocarcinoma (22). Primary mucinous adenocarcinoma is characterized by a highly invasive. When diagnosed, up to 40% of patients are reported to have metastatic disease. Lymphatic metastasis is the most common route, and cancer cells can be transferred to many parts of the body, including the lymph nodes in the ilium, obturator, the external iliac artery, and the common iliac artery. Cancer cells can also be transferred through blood, mainly to the liver, lungs, and bones. In addition, bladder mucinous adenocarcinoma can also appear directly in the prostate or posterior urethra through direct diffusion. Other studies have reported cases of bladder mucinous adenocarcinoma spreading to the ovary, uterus, abdominal wall, colon, and penis (23-25).
PBA is difficult for pathologists to diagnose because of the difficulty of differentiating it from secondary bladder involvement, resulting in adenocarcinomas of neighboring organs, especially in the prostate, colon, and female genital tract (26). A distinction made by a doctor is essential for determining stage or prognosis, and providing appropriate treatment. The tumor in our case is based on the extensive examination, eventually enabling the confirmation of the diagnosis of primary bladder tumor. The diagnostic stage of mucinous bladder adenocarcinoma mainly determines the prognosis. The pathogenesis of PBA is unknown. It is common for intestinal metaplasia to be found in the nearby mucosa of primary adenocarcinoma of the urinary bladder, which is largely considered as a precursor lesion (27,28). Although usually detected in the posterior wall and trigone, bladder adenocarcinoma can also develop in areas other than the bladder (29). However, through our review of the literature, we found that it is an exceedingly unusual that primary mucinous adenocarcinoma of the bladder is detected in the urethra, such as with our case. Typical clinical manifestations usually cannot be found in primary mucinous adenocarcinoma of the bladder. The most common symptoms include suprapubic pain, hematuria, dysuria, as well as bladder irritation (30). Difficulty in urinating and bladder irritation can be present in some patients (31). However, in our case, due to the location of the growth, the patient’s only symptoms were dysuria and urinary tract thinning, with no hematuria, suprapubic pain, or bladder irritation.
Ultrasound is the first choice for treatment in cases with positive symptoms of urinary pain, urgency, naked hematuria. Ultrasound images often lack specific expression for primary mucinous adenocarcinoma of the bladder. Although it can be shown that the adenocarcinoma is often not prominent to the bladder cavity, the basal area is actually broad and deep. CDFI can usually show blood flow signals. However, subjectivity and expertise of the examiner can often lead to limitations in the use of 2D ultrasound and CDFI. Ultrasound can only show tumor morphology and its CDFI, and nodal involvement and deeply infiltrating disease is unreliable via ultrasound (32). Multidetector (64-slice) CT scanning is the mainstay in radiological assessment, with sensitivity to bladder cancer at 85% and specificity at 94% (14). The size and shape of the tumor determine the detection. False negatives have a higher possibility to be seen in carcinoma in situ, and tumors <1 cm in size, and flat lesions, as well as patients who have recently undergone resection (32-34). At most centers, the intravenous urogram has been replaced by CT, which continues to serve as the modality of choice for examining hematuria. The 64-slice multidetector CT lacks reliability in ascertaining extensive locoregional disease, despite its high spatial resolution (35). This limitation results from interobserver variability, as well as difficulty in distinguishing the bladder muscle layers (32,36).
Given its rarity, the diagnosis of primary mucinous adenocarcinoma of the bladder still depends on the pathological examination. Hematoxylin-eosin (HE) staining is a recognized method of histopathology for observing histopathological structures. In this study, an adenoid structure was found in the mass by HE staining. IHC staining can differentiate a PBA from a secondary tumor involving the bladder, and most frequently, colorectal adenocarcinoma. CK20 is considered to be a marker of adenocarcinoma. CDX-2 and β-catenin are considered highly effective indicators of colorectal malignancies. GATA-3 is a marker of urothelial malignancies (37). In our case, manifestation of β-catenin weak positivity, CK20 positivity, and CDX-2 and GATA-3 negativity by IHC confirmed the primary mucinous adenocarcinoma of the bladder and excluded secondary spread from colorectal cancer. Syn, CgA, and CD56 are neuroendocrine markers, indicating neuroendocrine differentiation (38).
Our case is an exceedingly unusual case, in which the growth site is atypical, the tumor did not protrude into the bladder cavity, so ultrasound could not differentiate whether it came from the bladder or the urethra. In addition, our case lacks typical symptoms, so neither ultrasound nor CT could identify the origin of the disease. Only the location and characteristics of the lesion were identified and described, and the preliminary diagnosis of lesions was made. Even before the results of immunohistochemistry, clinicians have not completely ruled out the possibility that it is metastatic disease.
Guidelines for the optimal management options of PBA are unavailable. Surgical treatment has been reported to be the treatment of choice in previous studies (39), with pelvic lymphadenectomy and cystectomy the preferred choices. Transurethral resection of bladder tumor is not recommended (40). According to the National Comprehensive Cancer Network and European Association of Urology guidelines for bladder cancer, chemotherapy can be considered for advanced and metastatic adenocarcinoma of the bladder after surgery, and 5-fluorouracil-based chemotherapy for colorectal cancer is generally adopted (41,42). However, because of the low incidence of bladder cancer, there is no definite effective chemotherapy plan. Some previous studies have adopted the chemotherapy regimen for the treatment of transitional cell carcinoma (cisplatin + gemcitabine), but the curative effect is unknown due to the low number of cases (43,44). Considering that primary mucinous adenocarcinoma of the bladder has the characteristics of adenocarcinoma, most chemotherapy regimens for the treatment of gastrointestinal adenocarcinoma are recommended, such as FOLFOX regimen (oxaliplatin + calcium folinate + fluorouracil) or XELOX regimen (oxaliplatin combined with capecitabine). However, due to the rare nature of the tumor, the best treatment for primary mucinous adenocarcinoma of the bladder has not yet been determined (45,46). According to the imaging examinations, laboratory findings, pathological findings, and clinical stage of our case, 2 cycles of FOLFOX-6 regimen were performed. Through the follow-up, we learned that the patient was generally in good condition and her condition was stable, indicating that the treatment scheme given in the guidelines was effective. For the chemotherapy regimen of such patients, we recommend FOLFOX-6 regimen in consideration of the therapeutic effect of our patient.
In conclusion, primary mucinous adenocarcinoma of the bladder located at the bladder outlet and urethra is a rare disease entity. Unfortunately, there are not enough same cases. We have not summarized its typical clinical symptoms and imaging findings, but this case can still give us some reminders. For patients with atypical symptoms, in addition to careful ultrasound examination of the bladder, we should also pay attention to the changes of adjacent tissues of the bladder, which is very important for early diagnosis and treatment of this disease. The first choice of treatment for primary bladder mucinous adenocarcinoma is cystectomy and pelvic lymphadenectomy. Even though it is still controversial whether routine adjuvant therapy should be given after the operation, we recommend the chemotherapy regimen recommended by the guidelines. And close postoperative follow up is important to monitor for local and distant recurrence.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1547/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1547/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the national research committee and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2018;4:1553-68. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Heney NM, Ahmed S, Flanagan MJ, et al. Superficial bladder cancer: progression and recurrence. J Urol 1983;130:1083-6. [Crossref] [PubMed]
- Black PC, Brown GA, Dinney CP. The impact of variant histology on the outcome of bladder cancer treated with curative intent. Urol Oncol 2009;27:3-7. [Crossref] [PubMed]
- el-Mekresh MM, el-Baz MA, Abol-Enein H, et al. Primary adenocarcinoma of the urinary bladder: a report of 185 cases. Br J Urol 1998;82:206-12. [Crossref] [PubMed]
- Sigalas K, Tyritzis SI, Trigka E, et al. A male presenting with a primary mucinous bladder carcinoma: a case report. Cases J 2010;3:49. [Crossref] [PubMed]
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Cai Q, Chen Y, Xin S, et al. Temporal trends of bladder cancer incidence and mortality from 1990 to 2016 and projections to 2030. Transl Androl Urol 2020;9:153-65. [Crossref] [PubMed]
- Wilson TG, Pritchett TR, Lieskovsky G, et al. Primary adenocarcinoma of bladder. Urology 1991;38:223-6. [Crossref] [PubMed]
- Grignon DJ, Ro JY, Ayala AG, et al. Primary adenocarcinoma of the urinary bladder. A clinicopathologic analysis of 72 cases. Cancer 1991;67:2165-72. [Crossref] [PubMed]
- Baffigo G, Delicato G, Bianchi D, et al. Mucinous adenocarcinoma of the urinary bladder. Am J Case Rep 2012;13:99-101. [Crossref] [PubMed]
- Na YQ, Guo ZH. Practical Urology (2nd Edition). Beijing: People’s medical Publishing House, 2013:455-62.
- Wang ZX, Chen RB, Liu C, et al. Current Situation of diagnosis and treatment of adenocarcinoma of the urinary tract. Journal of Urology for Clinician (Electronic Version) 2016;8:1-6, 69.
- Tatli AM, Uysal M, Goksu SS, et al. Primary mucinous adenocarcinoma of the bladder: complete response with FOLFOX-4 regimen. Med Oncol 2012;29:1935-7. [Crossref] [PubMed]
- Tsironis G, Bamias A. Treating bladder adenocarcinoma. Transl Androl Urol 2018;7:S699-S701. [Crossref] [PubMed]
- Santos BM, de Souza JD, Lima RS, et al. Mucinous Bladder Adenocarcinoma: Case Report and Literature Review. Case Rep Urol 2015;2015:783109. [Crossref] [PubMed]
- Soares MJ, Neves T, Covita A, et al. Bladder adenocarcinoma. Case report. Arch Esp Urol 2008;61:828-31. [PubMed]
- Ojea Calvo A, Núñez López A, Domínguez Freire F, et al. Mucinous adenocarcinoma of the urachus. Actas Urol Esp 2003;27:142-6. [Crossref] [PubMed]
- Martínez-Piñeiro L, González-Peramato P, Hidalgo L, et al. Primary bladder adenocarcinoma: retrospective study of 11 cases and general review. Arch Esp Urol 1991;44:131-8. [PubMed]
- Di Lauro G, Iacono F, Ruffo A, et al. Presenting a case of a mucinous adenocarcinoma of an exstrophic bladder in an adult patient and a review of literature. BMC Surg 2013;13:S36. [Crossref] [PubMed]
- Zhang BY, Aguilar J, Yang M, et al. Mucinous metaplasia in urothelial tract may be the precancerous lesion of mucinous adenocarcinoma: report of two cases and review of literature. Int J Clin Exp Med 2014;7:285-9. [PubMed]
- Palmero Martí JL, Queipo Zaragozá JA, Bonillo García MA, et al. Mucinous adenocarcinoma of the bladder. Actas Urol Esp 2003;27:274-80. [Crossref] [PubMed]
- El-Ghobashy A, Ohadike C, Wilkinson N, et al. Recurrent urachal mucinous adenocarcinoma presenting as bilateral ovarian tumors on cesarean delivery. Int J Gynecol Cancer 2009;19:1539-41. [Crossref] [PubMed]
- Jo EJ, Choi CH, Bae DS, et al. Metastatic urachal carcinoma of the ovary. J Obstet Gynaecol Res 2011;37:1833-7. [Crossref] [PubMed]
- Neeli S, Prabha V, Alur S, et al. Penile metastasis from primay mucinous adenocarcinoma of bladder. Indian J Urol 2007;23:314-6. [Crossref] [PubMed]
- Melicow MM. Tumors of the urinary bladder: a clinico-pathological analysis of over 2500 specimens and biopsies. J Urol 1955;74:498-521. [Crossref] [PubMed]
- Cheng L, Montironi R, Bostwick DG. Villous adenoma of the urinary tract: a report of 23 cases, including 8 with coexistent adenocarcinoma. Am J Surg Pathol 1999;23:764-71. [Crossref] [PubMed]
- Zhong M, Gersbach E, Rohan SM, et al. Primary adenocarcinoma of the urinary bladder: differential diagnosis and clinical relevance. Arch Pathol Lab Med 2013;137:371-81. [Crossref] [PubMed]
- Pan X, Jin L, He T, et al. Mucinous adenocarcinoma of the bladder: A case report and review of the literature. Mol Clin Oncol 2016;5:447-8. [Crossref] [PubMed]
- Zaghloul MS, Nouh A, Nazmy M, et al. Long-term results of primary adenocarcinoma of the urinary bladder: a report on 192 patients. Urol Oncol 2006;24:13-20. [Crossref] [PubMed]
- Font A, Luque R, Villa JC, et al. The Challenge of Managing Bladder Cancer and Upper Tract Urothelial Carcinoma: A Review with Treatment Recommendations from the Spanish Oncology Genitourinary Group (SOGUG). Target Oncol 2019;14:15-32. [Crossref] [PubMed]
- MacVicar AD. Bladder cancer staging. BJU Int 2000;86:111-22. [Crossref] [PubMed]
- Martingano P, Stacul F, Cavallaro M, et al. 64-Slice CT urography: 30 months of clinical experience. Radiol Med 2010;115:920-35. [Crossref] [PubMed]
- Wang LJ, Wong YC, Ng KF, et al. Tumor characteristics of urothelial carcinoma on multidetector computerized tomography urography. J Urol 2010;183:2154-60. [Crossref] [PubMed]
- Paik ML, Scolieri MJ, Brown SL, et al. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol 2000;163:1693-6. [Crossref] [PubMed]
- Tritschler S, Mosler C, Tilki D, et al. Interobserver variability limits exact preoperative staging by computed tomography in bladder cancer. Urology 2012;79:1317-21. [Crossref] [PubMed]
- Wang HL, Lu DW, Yerian LM, et al. Immunohistochemical distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Am J Surg Pathol 2001;25:1380-7. [Crossref] [PubMed]
- Wang X, Li Y, Feng H, et al. Large cell neuroendocrine carcinoma of the ileocecal junction with well differentiation adenocarcinoma. Neuro Endocrinol Lett 2015;36:133-5. [PubMed]
- Aragon-Ching JB, Chung S, Paquette E, et al. Mucinous Signet-Ring Urachal Carcinoma of the Bladder: Case Report and Review of the Literature. Clin Genitourin Cancer 2017;15:e889-91. [Crossref] [PubMed]
- Ball MW, Nathan R, Gerayli F. Long-Term Response After Surgery and Adjuvant Chemoradiation for T4 Mucinous Adenocarcinoma of the Bladder: A Case Report and Review of the Literature. Clin Genitourin Cancer 2016;14:e225-7. [Crossref] [PubMed]
- Flaig TW, Spiess PE, Agarwal N, et al. NCCN Guidelines Insights: Bladder Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:1041-53. [Crossref] [PubMed]
- Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol 2021;79:82-104. [Crossref] [PubMed]
- Albers P, Park SI, Niegisch G, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment Ann Oncol 2011;22:288-94. [German Association of Urological Oncology (AUO) trial AB 20/99]. [Crossref] [PubMed]
- Maisch P, Retz M, Gschwend JE, et al. Clinical Practice Guidelines for Bladder Cancer: A Systematic Review and Meta-Analysis Using the AGREE II Instrument. Urol Int 2021;105:31-40. [Crossref] [PubMed]
- Chang SS, Bochner BH, Chou R, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol 2017;198:552-9. [Crossref] [PubMed]
- Merseburger AS, Apolo AB, Chowdhury S, et al. SIU-ICUD recommendations on bladder cancer: systemic therapy for metastatic bladder cancer. World J Urol 2019;37:95-105. [Crossref] [PubMed]
(English Language Editor: R. Scott)



