
Surgical treatment of intermediate to high grade thymic neuroendocrine neoplasms: case series of five patients and literature review
Introduction
Thymic neuroendocrine neoplasms (Th-NENs) are extremely rare, with an age-adjusted annual incidence of 0.02 per 100,000 US population, and account for approximately 5% of thymic tumors and 0.4% of all neuroendocrine neoplasms (1,2). Th-NENs were first described by Rosai and Higa in 1972, and so far only a few hundred cases have been reported (3). Th-NENs tend to occur in men aged 40 to 50 years and their symptoms are occult. At the time of diagnosis, tumors are usually large and invasive at the time of diagnosis, with an average diameter of 7–8 cm. Almost half of Th-NENs are functional and the most common neuroendocrine symptoms include Cushing’s syndrome caused by ectopic adrenocorticotropic hormone production, acromegaly caused by growth hormone releasing hormone hypersecretion and hypertrophic osteoarthropathy (4), while carcinoid syndrome is rare. The most common cause of anterior mediastinal masses in patients with multiple endocrine neoplasia type 1 (MEN1) is Th-NENs, and 20% of Th-NENs are associated with MEN1, usually nonfunctional and more common in men (5,6).
According to the latest World Health Organization (WHO) classification of tumors of the thymus and mediastinum in 2021 (7), Th-NENs are divided into two categories. One category is neuroendocrine tumors, including typical carcinoid (TC) and atypical carcinoid (AC). The other category is neuroendocrine carcinomas, including small cell carcinoma (SCC), combined SCC and large cell neuroendocrine carcinoma (LCNEC). AC, LCNEC, and SCC are highly invasive malignancies with low complete surgical resection rate at first diagnosis (8). Due to the low incidence of Th-NENs, there are very few studies at present, and most of them are single-center retrospective studies with a small sample size. Currently, the optimal treatment strategy for Th-NENs, especially surgical treatment strategy, is uncertain. Little is known about the scope of resection, lymph node dissection, and prognostic factors. To improve clinicians’ understanding of Th-NENs, we report a case series of Th-NENs after surgical treatment and review the literature. We present the following article in accordance with the AME Case Series reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1150/rc).
Methods
Patients and perioperative management
Between 2008 and 2020, a total of five non-consecutive patients diagnosed with Th-NENs underwent surgical resection at the department of thoracic surgery of Beijing Hospital in Beijing, China. Clinical characteristics, imaging and pathological features, surgical methods and perioperative outcomes of these patients were retrospectively reviewed. Preoperative chest CT, abdominal ultrasound, cranial magnetic resonance image (MRI) or CT, and whole body bone scan were performed to evaluate tumor stage. Tumor markers tested before surgery included embryonic antigen, CA19-9, CA125, CA153, squamous cell carcinoma antigen, neuron specific enolase and alpha fetoprotein. Arterial blood gas, pulmonary function and echocardiography were performed to assess surgical tolerance.
The pathological diagnosis criteria in our study was the 2021 WHO Classification of tumors of the thymus and mediastinum (7).Tumor stage was assessed by Masaoka-Koga staging system (9) and 8th edition of tumor-node-metastasis (TNM) classification of thymic epithelial tumors (10). Before surgery, all the patients underwent electromyography and neurological evaluation to assess whether they had myasthenia gravis and neuroendocrine symptoms.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Beijing Hospital (No. 2022BJYYEC-169-01). Individual consent for this retrospective analysis was waived.
Surgical techniques
All the patients underwent combined intravenous and inhalation general anesthesia with double-lumen endotracheal intubation. Surgical approaches included video-assisted thoracoscopic surgery (VATS) and median sternotomy. For VATS group, we made a 1-cm incision in sixth intercostal space along midaxillary line as the observation port and a 2-cm incision in the fourth intercostal space along the anterior axillary line as the operation port. All patients underwent extended thymectomy, which included tumor and thymus, pericardium fat, cervical root fat, and mediastinal fat around bilateral cardio-diaphragmatic angle. Lymph nodes around the cervical roots, bilateral phrenic nerves, trachea and mediastinum were dissected. At the end of the surgery, one or two drainage tubes were placed in the mediastinal area.
Follow-up and efficacy evaluation
Complications and postoperative 90-day mortality were recorded based on the definitions of General Thoracic Surgery Database of the Society of Thoracic Surgeons (11). After discharge, all the patients were followed up. Chest CT, abdominal ultrasound and blood tumor marker were reviewed every 3 months in the first 2 years after surgery, every 6 months in the next 3 years, and then every 1 year after 5 years of surgery. Cranial MRI and whole body bone scan were reviewed annually or when there were corresponding symptoms. Follow-up time and survival time were calculated from the date of surgery, and the last follow-up date was January 24, 2022.
Literature review
The term “thymic”, “thymus”, “mediastinal”, “neuroendocrine neoplasm”, “neuroendocrine carcinoma”, “neuroendocrine tumor”, “carcinoid”, “large cell neuroendocrine carcinoma”, and “small cell carcinoma” were searched in PubMed and Chinese literature database. Literatures related to surgical treatment of Th-NENs were collected, and clinical characteristic, surgical method, perioperative treatment, recurrence pattern, and survival data reported in these literatures were summarized.
Statistical analysis
Statistical analyses were performed using SPSS version 22.0 software. Continuous variables were presented as mean values ± standard deviation or median and range. Categorical variables were presented as numbers and percentages.
Results
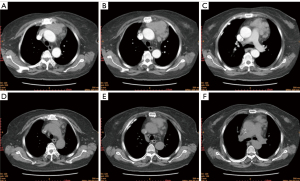
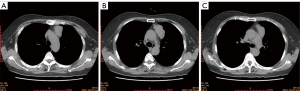
Five patients in this study were numbered as cases 1 to 5. There were three males and two females, and mean age was 53.6 (range, 44–66) years. Three patients complained of cough, chest pain and chest tightness respectively, and two patients were asymptomatic. No myasthenia gravis or neuroendocrine symptoms were found in all five patients. The average maximum tumor diameter is 57.8 (range, 45–84) mm. Case 1 to 3 were diagnosed with AC and case 4 to 5 were diagnosed with LCNEC. Lymph node or distant metastasis was found in case 2 and case 4 before surgery. According to Masaoka-Koga staging system, two patients were stage II-b, one patient was stage III-a, and two patients were stage IV-b. TNM staging results and other clinical characteristics were shown in Table 1. Typical chest CT images of LCNEC (case 5) and AC (case 3) in this case series were shown in Figures 1,2, respectively.
Table 1
| Case number | Age (years) | Gender | Pathologic type/IHC characteristics | Symptom at first visit | Neuroendocrine symptom | Blood tumor marker | Tumor location and size (mm) | Extent of tumor invasion | Metastasis | Masaoka-Koga stage/TNM stage |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | Male | AC/Ki-67 (5%+), Syn (+++), CgA (+++), NSE (+) | Persistent pain along the sternum | No | Normal | Anterior mediastinum, 45×40 | Perithymic adipose tissue | No | II-b/T1aN0M0, I |
| 2 | 48 | Male | AC/Ki-67 (30%+), Syn (+++), CgA (+), CD56 (++) | Asymptomatic, found enlarged supraclavicular lymph nodes occasionally | No | Normal | Anterior mediastinum, 50×46×33 | Adhesion to the wall of the left innominate vein | Mediastinal lymph nodes (4/4), cervical lymph nodes (3/3) | IV-b/T1bN2M0, IV-b |
| 3 | 66 | Female | AC/Ki-67 (10%+), Syn (+++), CgA (+), CD56 (+++), NSE (+++) | Chest distress | No | Normal | Anterior mediastinum, 50×50×26 | Perithymic adipose tissue | No | II-b/T1aN0M0, I |
| 4 | 45 | Male | LCNEC/Ki-67 (40%+), Syn (+), CgA (++), NSE (++) | Asymptomatic, found during physical examination | No | NSE increased (17.77 ng/mL) | Middle mediastinum, 60×44×44 | Invasion of right phrenic nerve, hilum, pericardium, superior vena cava, inferior vena cava, and right atrium | Mediastinal lymph nodes, sternum, and bilateral lung | IV-b/T4N2M1b, IV-b |
| 5 | 65 | Female | LCNEC, concurrent with ovarian cancer/Syn (++), CgA (+) | Cough | No | Normal | Anterior mediastinum, 84×55×47 | Perithymic adipose tissue and pericardium | No | III-a/T2N0M0, II |
Th-NENs, thymic neuroendocrine neoplasms; IHC, immunohistochemical; TNM, tumor-node-metastasis; AC, atypical carcinoid; NSE, neuron specific enolase; LCNEC, large cell neuroendocrine carcinoma.
Case 5 received preoperative chemotherapy, case 2 received preoperative sequence chemoradiotherapy, and the other three patients underwent surgery directly. Case 3 and case 5 underwent extended thymectomy via VATS, case 1 and case 2 underwent extended thymectomy via median sternotomy, and case 4 underwent resection of anterior mediastinal tumor, sternal metastases, superior vena cava and partial right atrium via median sternotomy and cardiopulmonary bypass. R0 resection was achieved in 80% (4/5) of patients. The mean operation time was (166±73) (range, 90–265) minutes, mean pleural drainage was (598±338) (range, 170–1,010) mL, median thoracic drainage duration was 3 (range, 2–4) days, and median postoperative hospital stay was 6 (range, 4–14) days. Case 4 experienced intraoperative blood transfusion. There was no postoperative 90-day complication and death. Case 4 and case 5 received adjuvant chemotherapy and case 1 received adjuvant radiotherapy.
The median follow-up time was 49 (range, 4–134) months. Case 1, diagnosed with stage II-b AC, had bilateral axillary lymph node metastases 116 months after surgery and was still alive after somatostatin analogue therapy. Case 2, diagnosed with stage IV-b AC, had multiple bone metastases and received radiotherapy 37 months after surgery. Case 3, diagnosed with stage II-b AC, had no recurrence 52 months after surgery. Case 4, diagnosed with stage IV-b LCNEC, had brain and lung metastases during postoperative chemotherapy and survived only 4 months after surgery. Case 5, diagnosed with stage III-a LCNEC concurrent with ovarian cancer, had no recurrence of Th-NENs, but metastases of ovarian cancer occurred 2 months after surgery, and survived only 8 months after surgery. Median disease-free survival was 37 (range, 0–134) months, and median overall survival (mOS) was 49 (range, 4–134) months. Treatment and outcome of five patients were shown in Table 2.
Table 2
| Case number | Preoperative treatment | Surgery | Surgical margin | Postoperative complication | Postoperative treatment | DFS (mo) | Recurrence site | Subsequent treatment | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | No | Extended Tx via median sternotomy | R0 | No | Chest RT (60 Gy) | 116 | Bilateral axillary lymph node metastases | Sandostatin LAR once a month for a year, and everolimus for maintenance therapy | 134, alive |
| 2 | Two cycles of CT (etoposide + cisplatin), chest RT (30 Gy), efficacy: PR | Extended Tx via median sternotomy | R0 | No | No | 37 | Multiple bone metastases | RT | 49, loss to follow-up |
| 3 | No | Extended Tx via VATS | R0 | No | No | 52+ | No | No | 52, alive |
| 4 | No | Resection of anterior mediastinal tumor, sternal metastases, superior vena cava and partial right atrium via median sternotomy and cardiopulmonary bypass | R2, residual tumor in the right atrium | No | Two cycles of CT (irinotecan + cisplatin) | 0 | Brain and lung metastases were detected on postoperative CT | One cycle of CT (irinotecan + cisplatin), mannitol for hydrocephalus | 4, dead |
| 5 | Four cycles of CT (paclitaxel + carboplatin), efficacy: SD | Extended Tx via VATS, ovarian cancer was not removed simultaneously | R0 | No | Two cycles of CT (paclitaxel + carboplatin) | 8 | No recurrence of Th-NENs; ileocecal and pelvic metastases of ovarian cancer occurred 2 mo after surgery | Right hemicolectomy, two cycles of CT (paclitaxel + carboplatin) | 8, dead |
Th-NENs, thymic neuroendocrine neoplasms; DFS, disease-free survival; mo, months; OS, overall survival; CT, chemotherapy; RT, radiotherapy; SD, stable disease; Tx, thymectomy; VATS, video-assisted thoracoscopic surgery; R0, no residual tumor; R2, macroscopic residual tumor.
A total of 22 original studies related to surgical treatment of Th-NENs were retrieved, as shown in Table 3.
Table 3
| Author [published year] | Volume | Gender (male/female) | Age (years), range | Neuroendocrine symptom | Pathology | Surgery | R0 resection rate (%) | Postoperative therapy | Recurrence DFS/OS (mo) | 5-y/10-y OS (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| de Montpréville (12) [1996] | 14 | 11/3 | 35–71 | Cushing (n=1), MEN1 (n=1) | AC (n=14) | Modality: total Tx (n=4), subtotal Tx (n=4), debulking surgery (n=1), biopsy (n=5); approach: NA | NA | CRT (n=4), RT (n=4) | Local progression (n=5), LR (n=3), DM (n=8); DFS: NA; OS: 1–109 | 31/0, 3-y OS: 46 |
| Fukai (13) [1999] | 15 | 10/5 | 19–73 | Cushing (n=2), MG (n=1) | TC (n=1), NEC (n=14) | Modality: total Tx (n=13), subtotal Tx (n=1), biopsy (n=1); approach: NA | 87 (13/15) | CRT (n=1), RT (n=7), CT (n=1) | DM (n=10), no recurrence (n=1), unknown (n=4); DFS: 4–99; OS: 15–127 | 33/7 |
| Moran (14) [2000] | 80 | 59/21 | 16–100 | Cushing (6%), endocrine abnormalities (16%), no other details | Low-grade (well differentiated) (n=29), intermediate grade (moderately differentiated) (n=36), high-grade (poorly differentiated) (n=15) | Modality: S (n=80); approach: NA | 100 (80/80) | NA | Metastatic sites: lymph nodes (16 cases), lung (11 cases), bone (3 cases), and esophagus, chest wall, and liver (1 case each); 5-y DFS: low-grade: 50%, intermediate grade: 20%, high-grade: 0% | 28/10 |
| Gal (15) [2001] | 10 | 7/3 | 36–77 | Cushing (n=3), MEN1 (n=1) | TC (n=2), AC (n=6), SCC (n=2) | Modality: total Tx (n=1), extended Tx (n=8), biopsy (n=1); approach: NA | NA | CRT (n=3), RT (n=2) | Intrathoracic recurrence (n=5), DM (n=8); mOS: TC: 102, AC: 63, SCC: 19 | NA |
| Tiffet (16) [2003] | 12 | 8/4 | 35–78 | Cushing (n=1), MEN1 (n=2) | TC (n=3), AC (n=6), SCC (n=1), LCNEC (n=2) | Modality: S (n=11), biopsy (n=1); approach: NA | 75 (9/12) | CRT (n=1), RT (n=3), CT (n=1) | LR (n=6), DM (n=9); OS: 1–86 | 50/NA |
| Cardillo (17) [2010] | 19 | 14/5 | 31–69 | eACTH (n=6), prolactin secreting (n=2), hyperparathyroidism (n=1) | TC (n=8), AC (n=6), LCNEC (n=5) | Modality: total Tx (n=7), extended Tx (n=12); approach: NA | 100 (19/19) | RT (n=8) | LR and re-surgery (n=2); DFS: 25–35; OS: 8–153 | 92/70 |
| Liang (18) [2011] | 5 | 4/1 | 26–78 | None | SCC (n=5) | Modality: radical resection (n=1), palliative resection (n=1), biopsy (n=3); approach: NA | 20 (1/5) | CRT (n=5) | Local progression and liver metastasis (n=1), DM (n=1); OS: NA | NA |
| Ahn (19) [2012] | 21 | 15/6 | 20–72 | Cushing (n=3) | AC (n=18), LCNEC (n=3) | Modality: S (n=21), no other details; approach: NA | 81(17/21) | CRT (n=8), RT (n=9), CT (n=3) | LR (n=2), DM (n=3), LR + DM (n=3); OS: 3–143 | 14/5 |
| Crona (20) [2013] | 28 | 22/6 | 19–64 | eACTH (n=4), Cushing (n=2), MEN1 (n=6) | NA | Modality: S (n=21), RT + S (n=1); approach: NA | 11 (3/28) | RT (n=18), CT (n=28) | DM (n=19); OS: 6–233 | 79/41 |
| Song (21) [2014] | 22 | 14/8 | 24–70 | NA | TC (n=3), AC (n=10), SCC (n=5), LCNE1C (n=4) | Modality: S (n=10), RT/CT (n=12); approach: NA | NA | CRT (n=2), RT (n=6) | DM (n=13), OS: 6–154; mOS: 59 | 45.5/NA |
| Su (22) [2015] | 12 | 9/3 | 29–76 | None | AC (n=12) | Modality: S (n=9), CT + S (n=1), biopsy (n=2); extended Tx (n=10); approach: sternotomy (n=5), thoracotomy (n=2), VATS (n=3) | 67 (8/12) | CRT (n=3), RT (n=2), CT (n=3) | Recurrence: 60% (6/10), mean disease progression time: 25.6 (range, 6–39); disease progression (n=8); OS: 6–46 | NA |
| Filosso (8) [2015] | 205 | 155/47 | 19–82 | NA | TC (n=49), AC (n=71), LCNEC and SCC (n=49), carcinoid NOS (n=9) | Modality: S (n=132), CT + S (n=19), RT + S (n=6); approach: sternotomy (n=95), thoracotomy (n=20), combined (n=2), clamshell (n=8), VATS or robotic (n=7) | 54 (52/96) | RT (n=70), CT (n=31) | Recurrence: 38% (36/94), no other details; mOS: 90 (95% CI: 81.48–not reached) | 68/39 |
| Chen (23) [2016] | 26 | 18/8 | 13–75 | Cushing (n=7), MEN1 (n=1) | TC (n=8), AC (n=12), SCC (n=5), LCNEC (n=1) | Modality: S (n=26); approach: sternotomy (n=18), thoracotomy (n=6), combined (n=2) | 85 (22/26) | CRT (n=9), RT (n=5), CT (n=2) | Recurrence: 34.6% (9/26), no other details; mOS: 51 (95% CI: 48.5–53.5) | 44.7/NA |
| Zhao (24) [2017] | 56 | 47/9 | Mean age: 54.3 | NA | TC + AC (n=56) | Modality: S (n=54), biopsy (n=2); approach: NA | 54 (30/56) | RT (n=37), CT (n=32) | LR (n=11), DM (n=22); 5-y DFS: 37.5%; 10-y DFS: 16.5%; mDFS: 55.7±7.0; mOS: 105.0±9.2 | 80.7/51.9 |
| Ye (25) [2017] | 9 | 4/5 | 28–72 | eACTH (n=1), MEN1 (n=9) | NA | Modality: S (n=8); approach: NA | NA | CRT (n=3), RT (n=2), CT (n=3) | Recurrence: 78% (7/9), no other details; mOS: 26.4 (95% CI: 12–70.8) | NA |
| Ose (26) [2018] | 30 | 25/5 | 17–80 | MEN1 (n=3) | TC (n=7), AC (n=11), SCC (n=9), LCNEC (n=3) | Modality: S (n=28); biopsy (n=2); approach: sternotomy (n=23), thoracotomy (n=3), combined (n=2), VATS (n=2) | 90 (27/30) | CRT (n=3), RT (n=6), somatostatin analogue (n=1) | LR (n=4), DM (n=7), LR + DM (n=3); 5-y DFS: 48%; 10-y DFS: 29%; OS: 1–208 | 77/35; 5-y CSS: 90 |
| Zaleski (27) [2021] | 27 | 21/6 | 20-89 | NA | TC (n=9), AC (n=18) | Modality: S (n=27); approach: NA | NA | NA | NA | 53/18 |
| Wu (28) [2021] | 21 | 18/3 | 21–72 | MEN1 (n=1) | AC (n=11), SCC (n=9), LCNEC (n=1) | Modality: S (n=4), S + CRT (n=3); approach: NA | NA | NA | OS: 1–115 | NA |
| Han (29) [2021] | 25 | 18/7 | 23–67 | Cushing (n=1), MEN1 (n=2) | TC (n=1), AC (n=24) | Modality: S (n=18), S + RT (n=2), S + CT (n=5); approach: NA | NA | NA | DM (n=3); OS: 1–72 | NA |
| Fang (30) [2021] | 146 | 104/42 | Mean age: 51.7 | eACTH (n=14), MEN1 (n=4), MG (n=2) | TC (n=54), AC (n=53), SCC (n=18), LCNEC (n=6), poorly differentiated carcinoma not specifically classified (n=15) | Modality: S (n=116), CT + S (n=12), CRT + S (n=7), CRT + somatostatin analogue + S (n=2); approach: sternotomy (n=91), thoracotomy (n=19), clam-shell (n=7), combined (n=4), VATS or robotic (n=11) | 75 (103/137) | CRT (n=34), RT (n=55), CT (n=9) | TC: LR (36.8%), pleural recurrence (5.3%), DM (57.9%); AC: LR 16.7%), pleural recurrence (16.7%), DM (66.7%); poorly differentiated carcinoma: LR (16.7%), pleural recurrence (8.3%), DM (75%); OS: NA | 70.1/40.4 |
| Gaur (31) [2010], a SEER database analysis | 160 | 116/44 | Median age: 57 | NA | Well differentiated (n=93), moderately differentiated (n=16), poorly differentiated (n=9), anaplastic tumors (n=10), unknown (n=30), mixed histology (n=2) | Modality: S (n=109); approach: NA | NA | NA | mOS: 64 (95% CI: 50–78); mOS for patients with localized, regional, and distant disease were 110, 59, and 35 mo | 53/NA; 5-y OS: localized: 80, regional: 48, distant disease: 31 |
| Sullivan (2) [2017], a SEER database analysis | 254 | 177/77 | Median age: 59 | NA | NA | Modality: S (n=139); approach: NA | NA | NA | mOS: all patients: 73 (95% CI: 57–89), surgical treatment group: 109 (95% CI: 90–128), non-surgical treatment group: 46 (95% CI: 35–57) | 56/26; 5-y OS rate: surgical treatment group: 74; non-surgical treatment group: 34 |
Th-NENs, thymic neuroendocrine neoplasms; R0, no residual tumor; DFS, disease-free survival; OS, overall survival; mo, months; y, year; Cushing, Cushing’s syndrome; MEN1, multiple endocrine neoplasia type 1; AC, atypical carcinoid; Tx, thymectomy; NA, not available; CRT, chemotherapy and radiotherapy; RT, radiotherapy; LR, local recurrence; DM, distant metastases; MG, myasthenia gravis; TC, typical carcinoid; NEC, neuroendocrine carcinoma; CT, chemotherapy; SCC, small cell carcinoma; mOS, median overall survival time; LCNEC, large cell neuroendocrine carcinoma; S, surgery; eACTH, ectopic ACTH secreting; VATS, video-assisted thoracoscopic surgery; CSS, cancer-specific survival; NOS, not otherwise specified; CI, confidence interval; mDFS, median disease-free survival; SEER, Surveillance, Epidemiology, and End Results.
Discussion
About one-third of Th-NENs are asymptomatic patients found during chest imaging examination. Symptoms of Th-NENs include cough, chest pain, dyspnea, superior vena cava syndrome, hoarseness and hemidiaphragm lifting. In our group, the average maximum diameter of tumor was over 5 cm, 60% (3/5) patients were asymptomatic, 40% (2/5) patients complained of chest tightness or chest pain, and no patients had myasthenia gravis or other neuroendocrine symptoms. Pathologically, all tumors had invaded perithymic adipose and organs, and 40% had lymph nodes or distant metastasis. There was 1 case of LCNEC, whose tumor had invaded the sternum, chest wall, vena cava and right atrium, with multiple metastases of mediastinal lymph node and lung at initial diagnosis. According to the literatures, almost half of Th-NENs patients are already inoperable locally advanced or metastatic, and common metastatic sites include lymph nodes, lung, liver, bone, pleura, and pericardium (2,8,31).
The chest CT features of Th-NENs are the lobulated mass located in the region of thymus, with uneven enhancement areas and a central low-density area due to hemorrhage and necrosis, especially in cases with large lesions. Contrast-enhanced CT showed punctate and malnourished calcification. in our group, Th-NENs were characterized by large, irregular solid masses, and mediastinal lymph node enlargement only occurred in 40% (2/5) of patients. Enhanced MRI is an effective method to detect liver and brain metastases. Somatostatin receptor positron emission tomography, usually using 68Ga-DOTA-conjugated peptides, can be used to detect local invasion and distant metastasis for various neuroendocrine neoplasms, as well as to screen patients for peptide receptor radionuclide therapy (32). Since none of the patients in our group had neuroendocrine symptoms, somatostatin receptor PET was not performed. The neuroendocrine function of Th-NENs was poorly understood at the time, and now we believe that serological and functional imaging screening for Th-NENs should be conducted more aggressively before surgery.
Surgical resection continues to be the mainstay in the treatment of Th-NENs and complete resection is an important prognostic factor (2,8). Radical resection is recommended for all resectable and potentially resectable Th-NENs, and the tumor, thymus and surrounding invaded organs (such as mediastinal pleura, lung, pericardium, etc.) should be completely removed. Adjacent mediastinal vessels (e.g., superior vena cava and its branches, brachiocephalic arteries, etc.) should be carefully assessed before surgery, and patients with severe vascular invasion may need vascular resection and reconstruction. Potential intrathoracic metastases (e.g., parietal pleura, visceral pleura, etc.) should be explored during surgery and, if present, removed as completely as possible. Due to the high invasiveness of Th-NENs, some patients only receive biopsy surgery. The reporting of surgical procedure and outcome in clinical studies remains to be standardized. Among the literature retrieved in this study, only 40% (9/22) reported the surgical approach and scope of resection, and less than 60% (13/22) reported the R0 resection rate. In our group, all patients underwent extended thymectomy and the R0 resection rate was 80% (4/5), among which 2 patients achieved radical resection after neoadjuvant chemotherapy.
Nearly 50% of Th-NENs have lymph node involvement at the time of surgical resection, and lymph node dissection is strongly recommended for accurate staging and better prognosis (33,34). However, few clinical studies have specifically reported the details of lymph node dissection. At present, due to insufficient data on lymph node dissection of Th-NENs, and there is no consensus on the number and scope of lymph node dissection. In our current clinical practice, lymph nodes around the phrenic nerve, innominate vein, superior vena cava, trachea and cervical root are recommended to be removed. In our study, lymph nodes around cervical root and mediastinum were removed in three patients, and lymph node dissection was not performed in two patients. Inadequate lymph node dissection may be one of the important factors contributing to poor prognosis after surgery. Therefore, in the future, the surgical scope of Th-NENs, especially the scope and number of lymph node dissection, should be fully studied and discussed.
In terms of surgical approach, median sternotomy is preferred for patients with large tumors and tumor invasion of important organs, and lateral thoracotomy is also alternative for patients with tumors located on one side of the thorax, or for patients with difficulty with a median sternotomy. In recent years, VATS thymectomy via intercostal or subxiphoid incisions has been more and more widely used and is suitable for patients with tumors less than 5 cm in diameter and no obvious tumor invasion. In our study, 40% (2/5) patients underwent VATS extended thymectomy via left intercostal incisions, one with a diameter of 5cm and the other with a diameter of 8 cm, and both patients achieved R0 resection. Therefore, we believe that tumor size is not the most important determining factor for minimally invasive surgery, and the extent and severity of tumor invasion have a greater impact on the choice of surgical approach.
For locally advanced Th-NENs, the multidisciplinary treatment model (neoadjuvant chemotherapy and/or radiotherapy + surgery + adjuvant chemotherapy and/or radiotherapy) should be considered, but there is no consensus on whether induction therapy can increase surgical resection rate and improve prognosis. Two large-sample studies reported neoadjuvant chemotherapy and/or radiotherapy plus surgery, with an R0 resection rate of 54–75% and 5-year OS rate of 70% (8,30). In our study, case 2, diagnosed with AC with multiple mediastinal lymph node metastases, received 2 cycles of chemotherapy and radiotherapy and achieved partial remission (PR). Then case 2 underwent extended thymectomy via sternotomy. Case 5, diagnosed with LCNEC with high tumor burden and pericardium invasion, received 4 cycles of chemotherapy and achieved stable disease (SD). Then case 5 underwent extended thymectomy via VATS. R0 resection was achieved in both cases.
The 5-year OS rate and 10-year OS rate of Th-NENs are 53–80% and 26–52%, respectively, significantly worse than the prognosis of bronchopulmonary neuroendocrine neoplasms (2,8,24,31). The 5-year OS rate of high-grade Th-NENs (LCNEC and SCC) is less than 30%, which is significantly worse than that of TC and AC (30,35). The most common sites of postoperative recurrence were lymph nodes, lung, pleura, bone and liver (14,24,26). If the recurrence is local and can be radically resected, reoperation may be considered. In our group, mOS was 49 months, and metastatic sites included axillary lymph nodes, bone and lung. The OS of two LCNEC patients was only 4–8 months, while the OS of three AC patients achieved 49–134 months, reflecting that the prognosis of LCNEC was significantly worse than that of AC. Everolimus may be a therapeutic approach for recurrent Th-NENs. Anamaterou reported 4 cases of Th-NENs (including 3 cases treated with surgery and 1 case treated with octreotide) treated with everolimus after disease progression, with progress-free survival of 7–42 months (36). Case 1 in this study, who developed bilateral axillary lymph node metastases 116 months after extended thymectomy, was treated with sandostatin LAR for 1 year and everolimus for maintenance therapy, and has survived for more than 134 months. Due to the low incidence of Th-NENs, there is no validated specific staging system. Currently, it is still recommended to stage Th-NENs according to the Masaoka-Koga or TNM staging system. The most important prognostic factors include tumor size, tumor stage, grade of tumor differentiation, surgical resectability, and surgical margin (2,8,14,24,31,37). Tang et al. developed a nomogram prognostic model that could predict OS of thymic neuroendocrine tumors, consisting of T stage, tumor grade, surgical resectability, and whether the patient received chemotherapy, and found that their model had better authentication capability than Masaoka-Koga and TNM staging system (38).
Through literature review, most of the studies on Th-NENs are single-center and small-sample studies, and there are only a few multi-center and large-sample retrospective studies, with insufficient data on surgical methods, perioperative treatment and recurrence patterns. Currently, several important surgical issues, such as surgical method, scope of lymph node dissection, how to perform multidisciplinary treatment, and reoperation after recurrence, remain to be studied. The rarity of Th-NENs results in a small number of cases at a single center, making it difficult to conduct high-quality clinical studies. Therefore, it is urgent and important to establish a multicenter database, analyze the clinical, imaging, pathological and prognostic characteristics of each subtype of Th-NENs, evaluate the efficacy of different treatment modalities and surgical strategies, and finally establish a more effective treatment modality.
Since this study is a single-center report with a very small number of enrolled cases, the credibility of our conclusions is limited and needs to be verified by large sample study.
Conclusions
Th-NENs, especially high-grade Th-NENs, are very rare but extremely aggressive malignancies, and a large proportion of patients are already advanced stage at first diagnosis. Early diagnosis and surgical resection are the most important methods to improve prognosis. Radical resection is recommended for all resectable and potentially resectable Th-NENs, and lymph node dissection is recommended for accurate staging and improved prognosis. Median sternotomy is preferred for patients with large tumors and peripheral invasion, and minimally invasive surgery is optional for early-stage Th-NENs. For locally advanced Th-NENs, whether neoadjuvant therapy is effective remains to be studied. In the future, multi-center, large-sample clinical studies are urgently needed to explore better treatment modality.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1150/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1150/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1150/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1150/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Beijing Hospital (No. 2022BJYYEC-169-01). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Sullivan JL, Weksler B. Neuroendocrine Tumors of the Thymus: Analysis of Factors Affecting Survival in 254 Patients. Ann Thorac Surg 2017;103:935-9. [Crossref] [PubMed]
- Rosai J, Higa E. Mediastinal endocrine neoplasm, of probable thymic origin, related to carcinoid tumor. Clinicopathologic study of 8 cases. Cancer 1972;29:1061-74. [Crossref] [PubMed]
- Phan AT, Oberg K, Choi J, et al. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas 2010;39:784-98. [Crossref] [PubMed]
- Teh BT, McArdle J, Chan SP, et al. Clinicopathologic studies of thymic carcinoids in multiple endocrine neoplasia type 1. Medicine (Baltimore) 1997;76:21-9. [Crossref] [PubMed]
- Goudet P, Murat A, Cardot-Bauters C, et al. Thymic neuroendocrine tumors in multiple endocrine neoplasia type 1: a comparative study on 21 cases among a series of 761 MEN1 from the GTE (Groupe des Tumeurs Endocrines). World J Surg 2009;33:1197-207. [Crossref] [PubMed]
- Marx A, Chan JKC, Chalabreysse L, et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: What Is New in Thymic Epithelial, Germ Cell, and Mesenchymal Tumors? J Thorac Oncol 2022;17:200-13. [Crossref] [PubMed]
- Filosso PL, Yao X, Ahmad U, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg 2015;149:103-9.e2. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Wright CD, Edwards FHSociety of Thoracic Surgeons General Thoracic Surgery Database Task Force, et al. The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2007;83:893-4. [Crossref] [PubMed]
- de Montpréville VT, Macchiarini P, Dulmet E. Thymic neuroendocrine carcinoma (carcinoid): a clinicopathologic study of fourteen cases. J Thorac Cardiovasc Surg 1996;111:134-41. [Crossref] [PubMed]
- Fukai I, Masaoka A, Fujii Y, et al. Thymic neuroendocrine tumor (thymic carcinoid): a clinicopathologic study in 15 patients. Ann Thorac Surg 1999;67:208-11. [Crossref] [PubMed]
- Moran CA, Suster S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. A clinicopathologic analysis of 80 cases. Am J Clin Pathol 2000;114:100-10. [Crossref] [PubMed]
- Gal AA, Kornstein MJ, Cohen C, et al. Neuroendocrine tumors of the thymus: a clinicopathological and prognostic study. Ann Thorac Surg 2001;72:1179-82. [Crossref] [PubMed]
- Tiffet O, Nicholson AG, Ladas G, et al. A clinicopathologic study of 12 neuroendocrine tumors arising in the thymus. Chest 2003;124:141-6. [Crossref] [PubMed]
- Cardillo G, Treggiari S, Paul MA, et al. Primary neuroendocrine tumours of the thymus: a clinicopathologic and prognostic study in 19 patients. Eur J Cardiothorac Surg 2010;37:814-8. [Crossref] [PubMed]
- Liang HL, Liu JJ, Xue WP, et al. Thymic Small Cell Carcinoma: Clinical Analyses of 5 cases. The Practical Journal of Cancer 2011;26:401-3.
- Ahn S, Lee JJ, Ha SY, et al. Clinicopathological analysis of 21 thymic neuroendocrine tumors. Korean J Pathol 2012;46:221-5. [Crossref] [PubMed]
- Crona J, Björklund P, Welin S, et al. Treatment, prognostic markers and survival in thymic neuroendocrine tumours. a study from a single tertiary referral centre. Lung Cancer 2013;79:289-93. [Crossref] [PubMed]
- Song Z, Zhang Y. Primary neuroendocrine tumors of the thymus: Clinical review of 22 cases. Oncol Lett 2014;8:2125-9. [Crossref] [PubMed]
- Su K, Zou SM, Chen SL, et al. Clinical analysis of 12 cases of thymus atypical carcinoid. Oncology Progress 2015;13:317-21.
- Chen YY, Liu HS, Li SQ, et al. Neuroendocrine Tumors of the Thymus: Outcomes after Surgical Resection and Prognostic Factors. Medical Journal of Peking Union Medical College Hospital 2016;7:190-4.
- Zhao Y, Gu H, Fan L, et al. Comparison of clinical features and survival between thymic carcinoma and thymic carcinoid patients. Eur J Cardiothorac Surg 2017;52:33-8. [Crossref] [PubMed]
- Ye L, Wang W, Ospina NS, et al. Clinical features and prognosis of thymic neuroendocrine tumours associated with multiple endocrine neoplasia type 1: A single-centre study, systematic review and meta-analysis. Clin Endocrinol (Oxf) 2017;87:706-16. [Crossref] [PubMed]
- Ose N, Maeda H, Inoue M, et al. Results of treatment for thymic neuroendocrine tumours: multicentre clinicopathological study. Interact Cardiovasc Thorac Surg 2018;26:18-24. [Crossref] [PubMed]
- Zaleski M, Kalhor N, Moran CA. Typical and Atypical Carcinoid Tumors of the Mediastinum: A Biomarker Analysis of 27 Cases With Clinical Correlation. Int J Surg Pathol 2021;29:358-67. [Crossref] [PubMed]
- Wu ZD, Wang JC, Lin JQ, et al. Clinicopathological characteristics and prognosis of 21 patients with thymic neuroendocrine tumors. Zhonghua Bing Li Xue Za Zhi 2021;50:664-6. [PubMed]
- Han J, Gao XZ, Xu Y, et al. Clinicopathological features of thymic carcinoid tumor: a study of 25 cases. Zhonghua Bing Li Xue Za Zhi 2021;50:1260-2. [PubMed]
- Fang W, Filosso PL, Roden AC, et al. Clinicopathological features and current treatment outcomes of neuroendocrine thymic tumours. Eur J Cardiothorac Surg 2021;59:1004-13. [Crossref] [PubMed]
- Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010;251:1117-21. [Crossref] [PubMed]
- Bozkurt MF, Virgolini I, Balogova S, et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur J Nucl Med Mol Imaging 2017;44:1588-601. [Crossref] [PubMed]
- Filosso PL, Ruffini E, Solidoro P, et al. Neuroendocrine tumors of the thymus. J Thorac Dis 2017;9:S1484-90. [Crossref] [PubMed]
- Weksler B, Holden A, Sullivan JL. Impact of Positive Nodal Metastases in Patients with Thymic Carcinoma and Thymic Neuroendocrine Tumors. J Thorac Oncol 2015;10:1642-7. [Crossref] [PubMed]
- Ströbel P, Zettl A, Shilo K, et al. Tumor genetics and survival of thymic neuroendocrine neoplasms: a multi-institutional clinicopathologic study. Genes Chromosomes Cancer 2014;53:738-49. [Crossref] [PubMed]
- Lang M, Hackert T, Anamaterou C. Long-term effect of everolimus in recurrent thymic neuroendocrine neoplasia. Clin Endocrinol (Oxf) 2021;95:744-51. [Crossref] [PubMed]
- de Laat JM, Pieterman CR, van den Broek MF, et al. Natural course and survival of neuroendocrine tumors of thymus and lung in MEN1 patients. J Clin Endocrinol Metab 2014;99:3325-33. [Crossref] [PubMed]
- Tang JY, Gao HJ, Shi GD, et al. Development and validation of a nomogram prognostic model for patients with neuroendocrine tumors of the thymus. Thorac Cancer 2020;11:2457-64. [Crossref] [PubMed]


