
Efficacy of osimertinib in a metastatic lung adenocarcinoma patient harboring somatic EGFR delL747_S752 and germline BIM deletion polymorphism: a case report and literature review
Introduction
Exon 19 deletion (19del) and the exon 21 L858R point mutation are the most established predictive factor for the efficacy of epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) in patients with non-small cell lung cancer (NSCLC). EGFR 19dels comprise a heterogeneous group of genetic aberrations, including deletions, substitutions, and insertions. Different subtypes of 19dels exhibit heterogeneous response to EGFR-TKIs treatment. More than 50 subtypes of EGFR 19dels have been identified in NSCLC (1-7). delE746_A750 (65.9–72.3%), delL747_P753insS (6.1–8.1%) and delL747_T751 (5.8–7.7%) are the most frequent subtypes (2,4,5,7). Published literature have reported the associations between common subtypes of EGFR 19dels and survival outcomes in NSCLC patients who received EGFR-TKI therapy (1,2,4,5,7). However, the efficacy of EGFR-TKI in NSCLC patients harboring EGFR L747_S752 deletions (delL747_S752) as an uncommon subtype of 19dels remains elusive.
BIM, also known as B-cell chronic lymphocytic leukemia/lymphoma (Bcl-2)-like 11, encodes BCL2L11, which is a member of Bcl-2 protein family. BIM deletion polymorphism, resulting from the 2903-bp genomic deletion occurring in intron 2 of BIM gene, is commonly seen in East Asian and Hispanic patients with EGFR-mutant lung cancer with an incidence rate of 11.3–18.6% (8,9). Some studies reported that BIM deletion polymorphism predicted an unfavorable prognosis in EGFR-mutant NSCLC patients when treated with EGFR-TKIs (8,10-12), but other studies failed to find an association between the presence of BIM deletion polymorphism and the clinical outcome in these patients (13,14). Moreover, an array of studies have demonstrated that increased BIM at the RNA level enhances killing of NSCLC cells by the EGFR-TKIs, which contributes to the molecular mechanisms leading to tumor regression (15-18).
Here, we present a metastatic lung adenocarcinoma (LUAD) patient harboring EGFR delL747L_S752S and BIM deletion polymorphism who benefited from third-generation EGFR-TKI osimertinib with a progression-free survival (PFS) of 8 months. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1050/rc).
Case presentation
In August 2018, a 34-year-old female presented with fever at night and lower back pain. Ultrasound-guided biopsy of right supraclavicular lymph nodes revealed a metastatic carcinoma. She was diagnosed with stage IV LUAD with metastases to brain, bone, liver, right kidney gland, bilateral hilar lymph nodes, left and right supraclavicular lymph nodes, retroperitoneal lymph node and left external iliac lymph nodes. Plasma-based genotype was performed and revealed the presence of EGFR 19del (delL747_S752) with an allele frequency (AF) of 38.8% and BIM deletion polymorphism. The patient’s treatment history is shown in Figure 1. She was initially given with icotinib combined with zoledronic acid. Her symptoms of lower back pain and fever were subsequently relieved. On October 20, 2018, the patient was switched to oral osimertinib based on the findings from FLAURA trial reported in January 2018 (19) that osimertinib demonstrates a longer progression-free survival than gefitinib/erlotinib in Asian patients with EGFR-mutant advanced NSCLC. In March 2019, the chest and abdominal computed tomography (CT) scans showed partial response (PR) (>50% reduction) in the primary lung tumor and metastatic liver tumor, and the magnetic resonance imaging (MRI) showed complete remission of the brain metastases (Figure 2). In June 2019, the chest and abdominal CT scans showed the presence of nodular mass in bilateral lungs, the enlarged primary lung tumor and a metastatic liver tumor (Figure 2A,2B). The brain MRI showed a newly developed lesion in the right frontal lobe with a diameter of 2 mm (Figure 2C). The assessment of tumor response was progressive disease (PD) with a PFS of 8 months (Figure 1). We also reviewed the previously reported clinical outcomes to EGFR-TKIs in NSCLC patients harboring different 19dels, which are summarized in Table 1.
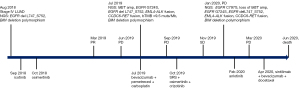
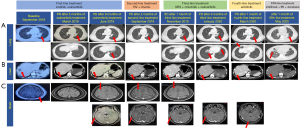
Table 1
| Publication | Year of publication | Clinical setting of EGFR-TKI treatment | EGFR 19dels | No. of patients | Median PFS or PFS in mo | P value |
|---|---|---|---|---|---|---|
| Chung et al. (5) | 2012 | First-line, second-line, third-line, or subsequent line gefitinib/erlotinib | Delta E746 | 219 | 9.8 | 0.665 |
| Delta E747 | 79 | 10.5 | ||||
| Non-LRE deletions | 10 | 5.9 (6.6–20.8) | ||||
| Delt751-i759insn | 1 | NA | ||||
| Delt751-e758 | 1 | NA | ||||
| S752-i759 | 1 | NA | ||||
| Delr748-P753 | 1 | 6.6 | ||||
| Delr748-S752 | 1 | 20.8 | ||||
| Dels752-I759 | 1 | 3.3 | ||||
| Dels752-I759 | 1 | 2.7 | ||||
| Dels752-i759insn | 1 | 5.9 | ||||
| Delt751-i759insd | 1 | 4.9 | ||||
| Delt751-i759insn | 1 | 4.8 | ||||
| Lee et al. (1) | 2013 | First-line gefitinib/erlotinib | Delta E746 | 46 | 14.2 | 0.021 |
| Delta L747 | 16 | 6.5 | ||||
| Dell747_p753inss | 10 | 6.5 (2.1–15.3) | 0.021 | |||
| Dele746_A750 | 42 | 12.4 | ||||
| Mix insertions/substitution | 12 | 22.3 (0.7–63) | ||||
| Dell747_k754insankg | 1 | 0.7 | ||||
| Dell747_a750insp | 1 | 1.5 | ||||
| Delt751_i759insn | 1 | 2.4 | ||||
| Dell747_a750insp | 1 | 5.7 | ||||
| Dele746_t751insv | 1 | 7.6 | ||||
| Delt751_i759inss | 1 | 13.3 | ||||
| Dele746_t751insi | 1 | 16.1 | ||||
| Dell747_a755insskg | 1 | 18.3 | ||||
| Dele746_t751insva | 1 | 22.3 | ||||
| Dell747_t751insp | 1 | 40.6 | ||||
| Dele746_s752insv | 1 | 43.1 | ||||
| Dele746_a750insap | 1 | 63 | ||||
| Non-LRE deletions | ||||||
| Delta T751 | 1 | 13.3 | ||||
| Delta T751 | 1 | NR | ||||
| Kaneda et al. (4) | 2014 | First-line, second-line or subsequent line gefitinib/erlotinib | Delta E746 | 11.7 | 0.022 | |
| Delta E747 | 10.0 | |||||
| With ins/sub | 10.0 | 0.024 | ||||
| Without ins/sub | 11.7 | |||||
| Zhao et al. (2) | 2020 | First-line gefitinib/erlotinib | Delta E746 | 78 | 12.1 | 0.816 |
| Delta L747 | 15 | 10.6 | ||||
| Delta L747 with insertions | 8 | 8.3 | 0.017 | |||
| Delta L747 without insertions | 7 | 15.0 | ||||
| Dele746_A750 | 72 | 12.1 | 0.795 | |||
| Dele746_a750ins | 6 | 10.0 | ||||
| Deletions with insertion | 14 | 9.5 | 0.102 | |||
| Deletions without insertion | 76 | 12.6 | ||||
| Peng et al. (3) | 2020 | First-line gefitinib/erlotinib | Uncommon 19delins | 93 | 19 | 0.0016 |
| Common19del (E746_A750) | 93 | 13 | ||||
| Dell747_p753inss | 23 | 18 | 0.58 | |||
| Dell747_a750insp | 24 | 20 | ||||
| Xu et al. (6) | 2020 | First-line gefitinib/erlotinib/icotinib | Delta E746 | 126 | 11.4 | <0.001 |
| Delta L747 | 62 | 17.2 | ||||
| Delta T51 or S752 | 6 | 2.9 | ||||
| Wang et al. (7) | 2021 | First-line gefitinib/erlotinib/icotinib | Del L747_T751delinsP | 6 | 18.7 | 0.035 |
| Others | 35 | 13.1 | ||||
| Our work | 2022 | First-line osimertinib | DelL747_S752 | 1 | 8 |
No., number; NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; PFS, progression-free survival; mo, month; delta E746, deletions starting from E746; delta L747, deletions starting from L747; ins/sub, insertions/substitutions; NR, the patient was still free from progression after taking EGFR-TKI for 2.4 months; TKI, tyrosine kinase inhibitor; NA, the PFS data of those patients was unavailable; delta T751, deletions starting at T751; Non-LRE, deletions not encompassing the entire amino acid string from L747 through E749; 19delins, exon 19 insertion-deletion variants; others, delL747_P753delinsS, L747_A750delinsP, and E746_S752delinsV.
The patient was treated with bevacizumab plus pemetrexed and carboplatin as second-line therapy on July 24, 2019. Meanwhile, next-generation sequencing (NGS) analysis using a panel covering 520 cancer-related genes (20) was performed on the plasma sample after failure of osimertinib treatment. The results revealed a blood tumor mutation burden (bTMB) of 9.5 mutations/Mb and the presence of new alterations, including EGFR G724S, MET amplification with a copy number of 4.0, EML4-ALK fusion and CCDC6-RET fusion. After three courses of the treatment, the brain MRI showed PD with newly developed metastases to the brain and enlarged tumor in right frontal lobe with a diameter of 4 mm (Figure 2C).
On October 8, 2019, she received stereotactic radiosurgery (SRS) as the definitive local therapy and was subsequently challenged with osimertinib combined with crizotinib. In November, 2019, the chest and abdominal CT scans showed shrinkage of the metastatic liver tumor (Figure 2B). The treatment response assessment was stable disease (SD). She had loss of appetite during treatment of osimertinib plus crizotinib. In January 2020, the chest CT scans demonstrated PD with enlarged bilateral lung metastatic lesions and newly developed lesions in the lower lobe of the left lung (Figure 2A,2C). At PD, NGS analysis was performed on plasma sample. NGS results showed the presence of new alterations, EGFR C797S and loss of MET amplification. She was administered with anlotinib as a single agent in February 2020. After one month of the treatment, the chest CT and brain MRI revealed newly developed metastatic lesions in bilateral lungs and brain metastatic tumors (Figure 2A,2C). The assessment of the response to fourth-line treatment was PD. In April, 2020, she was treated with sintilimab plus bevacizumab and docetaxel. The patient underwent nausea, vomiting, and muscle pain. After two months of the treatment, she succumbed to her disease in June 2020.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
To the best of our knowledge, we reported for the first time that a metastatic LUAD patient with EGFR delL747_S752 and BIM deletion polymorphism received osimertinib treatment.
Several studies have reported the association between subgroup of 19dels and clinical outcomes to EGFR-TKI in NSCLC patients, but have obtained conflicting results. A prior study reported by Chung et al. (5) is the first to subcategorize EGFR 19dels into three groups, deletions starting on codon E746 (delta E746), deletions starting on codon L747 (delta L747), deletions not involving the entire amino acid string from L747 through E749 (Non-LRE deletions). Chung et al. (5) and Zhao et al. (2) found that subgroup of 19dels was not associated with PFS in NSCLC patients treated with first-generation EGFR-TKIs. However, some studies demonstrated the presence of association between subgroup of 19dels and clinical outcome to EGFR-TKI in NSCLC patients. Lee et al. (1) revealed that delta L747 predicted a significantly shorter PFS compared to delta E746 (6.5 vs. 14.2 months) in NSCLC patients treated with first-generation EGFR-TKIs as first-line treatment. The similar results (10.0 vs. 11.7 months) were observed in a cohort of NSCLC patients treated with EGFR-TKIs as any line of first-generation EGFR-TKIs (4). In contrast, Xu et al (6) reported that delta L747 predicted a longer PFS than delta E746 (17.2 vs. 11.4 months). The controversial results may be attributed to several factors. Some studies (1-3,6) only included patients who were treated with EGFR-TKIs as fist-line setting, in contrast, others included patients using EGFR-TKIs as first-, second- or later-line treatment (4,5).
EGFR delL747_S752 is an uncommon subtype of 19dels, accounting for 0.6–3.6% of cases with EGFR 19dels (2,4,5). Due to its rarity, patients with this rare subtype are commonly pooled for investigating the clinical outcomes to EGFR-TKIs in NSCLCs. There are very few reports in the efficacy of EGFR-TKI against delL747_S752. The previous study (2) has demonstrated delta L747 with insertions (n=22) predicted a significantly shorter PFS than delta L747 without insertions (n=16) (8.3 vs. 15.0 months) in a cohort of 208 NSCLC patients treated with first-line EGFR-TKIs. Only one patient (6.25%, 1/16) harboring EGFR delL747_S752 was observed in delta L747 without insertions group (2). The true efficacy of EGFR-TKI in EGFR delL747_S752 patients could be masked by other patients. Evidence suggested that patients carrying EGFR delL747_S752 showed a good response (an objective response rate of 88.9%) to gefitinib or erlotinib in a small cohort (n=11) (5). In addition, a recent study shows subtypes of delta L747 with insertions impact on the efficacy of first-line EGFR-TKI that delL747_T751delinsP that predict a better prognosis (7). However, whether patients with EGFR delL747_S752 benefit from EGFR-TKIs has not been documented. In the present work, a metastatic LUAD patient harboring EGFR delL747_S752 and BIM deletion polymorphism obtained benefit from first-line osimertinib treatment with a PFS of 8 months. Moreover, EGFR G724S, MET amplification, EML4-ALK fusion and CCDC6-RET fusion were identified when she progressed from osimtertinib. These new alterations contributed to acquired resistance mechanisms to osimertinib treatment, which was consistent with a previous study indicating that EGFR G724S, MET amplification, ALK fusions and RET fusions were resistance mechanisms reported for osimertinib as first-line treatment (9,21).
In this work, the patient with somatic EGFR del747_S752 and germline BIM deletion polymorphism was given with icotinib in September 2018 obtaining PR to icotinib, and switched to osimertinib based on the findings of FLAURA trial reported in January 2018. The updated data on FLAURA trial published in January 2020 has revealed no difference of overall survival was observed in osimertinib versus gefitinib/erlotinib group for the Asian patients (22). Upon the previous and our work, we cannot conclude that osimertinib is more effective than icotinib based on the presence of the germline BIM deletion. Further in vitro, in vivo, and clinical trials studies are warranted to investigate whether osimertinib is more effective than icotinib in lung cancers with germline BIM deletion.
A recent study has revealed that regimens starting with afatinib and subsequently switched to osimertinib suppressed tumor development more efficiently than the opposite combination for EGFR variants according to preclinical assessment (23). These findings suggest that afatinib followed by osimertinib might be an efficacious treatment regimen for NSCLC patients with somatic EGFR delL747_S752 and concurrent germline BIM deletion polymorphism and further clinical trials are warranted.
There are some limitations associated with our study. First, since this is a case report, large cohort studies or clinical trials are necessary to validate the efficacy of osimertinib for metastatic LUAD patients with EGFR delL747_S752 and BIM deletion polymorphism. Second, BIM expression at the RNA level reported to contribute to molecular mechanisms leading to tumor regression was not investigated for the patient.
In conclusion, our work revealed that an EGFR delL747_S752/BIM deletion polymorphism double-positive LUAD patient obtained a PFS of 8 months with osimertinib treatment. Our work suggests that osimertinib might be a compromised treatment option for NSCLC patients with EGFR delL747_S752 and BIM deletion polymorphism. Further studies are needed to develop the more effective regimen for this small subset of NSCLCs, such as afatinib followed by osimertinib.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1050/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1050/coif). SJ declares that this study was supported by the project from the Shenzhen Science and Technology Program (Grant No. RCJC20200714114436049) and Cancer Hospital, Chinese Academy of Medical Sciences, Shenzhen Center/Shenzhen Cancer Hospital Research Project (No. SZ2020ZD006). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee VH, Tin VP, Choy TS, et al. Association of exon 19 and 21 EGFR mutation patterns with treatment outcome after first-line tyrosine kinase inhibitor in metastatic non-small-cell lung cancer. J Thorac Oncol 2013;8:1148-55. [Crossref] [PubMed]
- Zhao C, Jiang T, Li J, et al. The impact of EGFR exon 19 deletion subtypes on clinical outcomes in non-small cell lung cancer. Transl Lung Cancer Res 2020;9:1149-58. [Crossref] [PubMed]
- Peng X, Long X, Liu L, et al. Clinical impact of uncommon epidermal growth factor receptor exon 19 insertion-deletion variants on epidermal growth factor receptor-tyrosine kinase inhibitor efficacy in non-small-cell lung cancer. Eur J Cancer 2020;141:199-208. [Crossref] [PubMed]
- Kaneda T, Hata A, Tomioka H, et al. Possible differential EGFR-TKI efficacy among exon 19 deletional locations in EGFR-mutant non-small cell lung cancer. Lung Cancer 2014;86:213-8. [Crossref] [PubMed]
- Chung KP, Wu SG, Wu JY, et al. Clinical outcomes in non-small cell lung cancers harboring different exon 19 deletions in EGFR. Clin Cancer Res 2012;18:3470-7. [Crossref] [PubMed]
- Xu H, Li W, Yang G, et al. Heterogeneous Response to First-Generation Tyrosine Kinase Inhibitors in Non-Small-Cell Lung Cancers with Different EGFR Exon 19 Mutations. Target Oncol 2020;15:357-64. [Crossref] [PubMed]
- Wang Y, Zheng R, Hu P, et al. Patients harboring uncommon EGFR exon 19 deletion-insertion mutations respond well to first-generation EGFR inhibitors and osimeritinib upon acquisition of T790M. BMC Cancer 2021;21:1215. [Crossref] [PubMed]
- Wakabayashi Y, Masuda T, Fujitaka K, et al. Clinical significance of BIM deletion polymorphism in chemoradiotherapy for non-small cell lung cancer. Cancer Sci 2021;112:369-79. [Crossref] [PubMed]
- Zeng Y, Yu D, Tian W, et al. Resistance mechanisms to osimertinib and emerging therapeutic strategies in nonsmall cell lung cancer. Curr Opin Oncol 2022;34:54-65. [Crossref] [PubMed]
- Isobe K, Hata Y, Tochigi N, et al. Clinical significance of BIM deletion polymorphism in non-small-cell lung cancer with epidermal growth factor receptor mutation. J Thorac Oncol 2014;9:483-7. [Crossref] [PubMed]
- Zhao M, Zhang Y, Cai W, et al. The Bim deletion polymorphism clinical profile and its relation with tyrosine kinase inhibitor resistance in Chinese patients with non-small cell lung cancer. Cancer 2014;120:2299-307. [Crossref] [PubMed]
- Ng KP, Hillmer AM, Chuah CT, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med 2012;18:521-8. [Crossref] [PubMed]
- Liu SY, Zhou JY, Li WF, et al. Concomitant genetic alterations having greater impact on the clinical benefit of EGFR-TKIs in EGFR-mutant advanced NSCLC than BIM deletion polymorphism. Clin Transl Med 2020;10:337-45. [Crossref] [PubMed]
- Lee JY, Ku BM, Lim SH, et al. The BIM Deletion Polymorphism and its Clinical Implication in Patients with EGFR-Mutant Non-Small-Cell Lung Cancer Treated with EGFR Tyrosine Kinase Inhibitors. J Thorac Oncol 2015;10:903-9. [Crossref] [PubMed]
- Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med 2007;4:1669-79; discussion 80. [Crossref] [PubMed]
- Cragg MS, Kuroda J, Puthalakath H, et al. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med 2007;4:1681-89; discussion 90. [Crossref] [PubMed]
- Gong Y, Somwar R, Politi K, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med 2007;4:e294. [Crossref] [PubMed]
- Faber AC, Corcoran RB, Ebi H, et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov 2011;1:352-65. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Wang M, Chen X, Dai Y, et al. Concordance Study of a 520-Gene Next-Generation Sequencing-Based Genomic Profiling Assay of Tissue and Plasma Samples. Mol Diagn Ther 2022; [Crossref] [PubMed]
- Shaikh M, Shinde Y, Pawara R, et al. Emerging Approaches to Overcome Acquired Drug Resistance Obstacles to Osimertinib in Non-Small-Cell Lung Cancer. J Med Chem 2022;65:1008-46. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Ikeuchi H, Hirose T, Ikegami M, et al. Preclinical assessment of combination therapy of EGFR tyrosine kinase inhibitors in a highly heterogeneous tumor model. Oncogene 2022;41:2470-9. [Crossref] [PubMed]


