
Immunotherapy for combined pulmonary fibrosis and emphysema with advanced lung cancer: two case reports and a concise review
Introduction
Combined pulmonary fibrosis and emphysema (CPFE) with upper lobe emphysema and lower lobe fibrosis of the lung had been recognized as a unique disease that is distinct from emphysema or fibrosis (1,2). It is strongly correlated with males (approximately 90%) who are tobacco smokers or former smokers with a smoking history of more than 40 pack-years (strong association: 98%) and a mean age of 65–70 years (2,3). Significantly, patients with CPFE had an increased risk of lung cancer (3,4). CPFE with lung cancer (up to 46%) has been reported in the clinical characteristics and outcomes of a series of patients (5).
However, while CPFE has been the subject of case reports or short series, there have been limited studies that focused on the effect and safety of anti-tumor therapeutic modalities, such as surgical treatments, chemotherapy, and immunotherapy (6,7). While immune checkpoint inhibitors (ICIs) have shown efficacy in some advanced non-small cell lung cancers (NSCLCs), they can cause unique immune-related adverse effects. Currently, there are few studies on the safety of immunotherapy in patients with CPFE with advanced lung cancer (CPFE-LC) (6). Herein, we reported two cases of combination immunotherapy for advanced NSCLC with CPFE and reviewed published articles to determine the efficacy and safety of immunotherapy and combination therapy in CPFE-LC We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1526/rc).
Case presentation
The patient was a 64-year-old man who had a 40-pack-year history of smoking. Pulmonary function testing showed a decrease in the diffusion capacity of the lung for carbon monoxide of 39% of the predicted value, and a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio of 63.65% before surgery (Figure 1A,1B). He had a video-assisted thoracoscopic surgery (VATS) at another hospital and was diagnosed with squamous carcinoma (Figure 1C). Then the patient underwent stereotactic radiotherapy in different hospitals. In our hospital computed tomography (CT) revealed disease progression in the right upper lobe, a new mass in the left chest wall, and a combination of upper lobe emphysema and lower lobe fibrosis. The tumor, node, and metastasis classification (8th edition) was T4N2M1b (stage IV). Meanwhile, chest high-resolution computed tomography (HRCT) revealed ground-glass shadows and patchy consolidation with progressive fibrosis in the lower lung lobe.
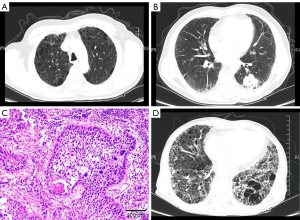
The patient complained of cough, dyspnea, and fever. We considered that the acute exacerbations of interstitial lung disease (AE-ILD) and radiation pneumonitis were induced by stereotactic radiotherapy. Therefore, we administered methylprednisolone 40 mg daily for one week and it was tapered as follows: 30 mg daily for 1 week, 20 mg daily for 1 week, 15 mg daily for 4 weeks, and 10 mg daily for 4 weeks. The consolidation and ground-glass shadows improved gradually. At that time, his oxygen saturation was 97–99% in room air. The performance status score decreased to two points after therapy, and he needed treatment for advanced lung cancer. According to the guidelines of the Chinese Society of Clinical Oncology (CSCO), the patient was finally treated with sintilimab, which is a programmed death-1 (PD-1) inhibitor, every 3 weeks for six cycles (200 mg, day 1). The initial treatment was a combination of sintilimab and gemcitabine. After two cycles of treatment, the patient was treated with immunotherapy monotherapy because of severe vomiting. A repeat scan showed that the pulmonary tumor had shrunk, and part of the metastasis had resolved entirely. However, the patient showed worsening respiratory symptoms with increased consolidation and ground-glass shadows, and an increased extent of honeycombing and other fibrosis on HRCT (>10%) (Figure 1D). We made a diagnosis of AE-ILD induced by immunotherapy and radiotherapy which was called radiation recall pneumonitis (RRP). With a tapering of the prednisolone dose, the patient developed the progressive fibrosing ILD phenotype. He was treated with pirfenidone, which is an oral anti-fibrotic and anti-inflammatory agent. Three months later, the patient died due to fibrosing of CPFE.
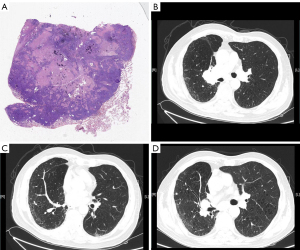
The second patient was a 74-year-old man with hypertension, coronary heart disease, chronic obstructive pulmonary disease, and a 50-pack-year smoking history who was admitted to our hospital for cough and dyspnea. He underwent radical surgery for lung cancer (for lung squamous cell carcinoma in the early stage with programmed death ligand-1 positive) two years before being admitted to our hospital (Figure 2A). CT revealed a tumor in the right hila, a nodule in the right middle lobe, liver tumors, and a combination of upper lobe emphysema and lower lobe fibrosis (Figure 2B,2C). The patient was diagnosed with lung cancer (T4NxM1) and CPFE. He received six cycles of a PD-1 inhibitor (sintilimab) (200 mg, day 1). His first 2-cycle treatment was with a combination of sintilimab with gemcitabine, and his 4-cycle treatment was with sintilimab monotherapy. CT revealed that the tumor had shrunk and the fibrosis of CPFE did not progress (Figure 2D). After 6 cycles of treatment, the patient stopped immunotherapy for personal reasons. Finally, the patient died of clinical progression caused by tumor invasion.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
As previously reported, CPFE is characterized by subnormal spirometry, severe impairment of gas exchange, high prevalence of pulmonary hypertension, and poor survival (8,9). Mortality was significant in CPFE with a reported median survival of between 2.1 and 8.5 years and 5-year survival of 38–55% (10). Because patients with ILD or emphysema are at an increased risk of developing lung cancer, especially in the case of male smokers, CPFE has also been associated with a higher risk of the development of lung cancer (up to 46%) and it is more prevalent than fibrosis in lung cancer patients. The previous study has shown that squamous cell carcinoma is the dominant type of cancer in patients with CPFE (5). Due to the lack of understanding of CPFE, the actual incidence of lung cancer in combination with CPFE may be high. Therefore, it is necessary to carry out comprehensive studies to better understand this complex combination.
Compared to lung cancer alone, the outcome of lung cancer with CPFE or ILD has been reported to be poor. Individuals with ILD have been excluded from most prospective clinical trials of lung cancer therapies because of the risk of acute exacerbations. Nevertheless, some retrospective analyses have summarized that acute exacerbations can develop during various cancer therapies, including surgery, radiotherapy, and pharmacotherapy in lung cancer with ILD (ILD-LC) (11-14). The current study provides a limited analysis of patients with CPFE-LC.
In our two case reports, we did not observe acute exacerbations in patients after surgery, and both had responses after the treatment of chemotherapy combined with immunotherapy. However, there were differences in the changing trend of ILD, progression was observed in case 1, and there was no change in case 2. Case 1 showed a worsening of respiratory symptoms with increased consolidation and ground-glass shadows, and an increased extent of honeycombing and other fibrosis on HRCT (>10%). He experienced AE-ILD after successful treatment with immunotherapy and developed a progressive-fibrosing phenotype. He died due to ILD with partial remission of lung cancer. However, case 2 achieved partial remission without any immune-related adverse events after immunotherapy, and the radiological pattern of CPFE was stabilized. The different changes in CPFE between the two patients may be associated with immunotherapy. Both of them received ICIs. ICIs alone or in combination with chemotherapy have been shown to improve overall survival (OS) compared to chemotherapy in patients who cannot benefit from targeted therapy (15). However, 7–13% of NSCLC patients treated with PD-1 axis inhibitors experienced grade three or higher toxicities. One serious manifestation was immune-mediated lung injury, usually ILD, which occurred in approximately 10% of patients receiving ICIs in the real world (16,17). ILD is known to lead to serious lung injury or life-threatening complications. Consequently, patients with CPFE-LC appeared to exhibit very challenging in immunotherapy.
There was a difference in therapies between the two cases, only case 1 had received radiation treatment and was considered suffering radiation pneumonitis or AE-ILD before receiving immunotherapy. In Teng’s opinion, ICIs may evoke RRP, which is a unique pattern of radiation-related toxicity in the patients’ previously irradiated fields (18). The choice of immunotherapy should be cautious in CPFE-LC with a history of radiation pneumonitis or AE-ILD.
Treatment of ICI-induced AE-ILD is based on systemic steroids. However, in patients with CPFE, emphysema is known to be associated with a higher risk of infection, such as bacterial pneumonia, and therefore it is difficult to treat it with glucocorticoids for 4–6 weeks. If a course of steroids does not reduce the severity of the initial symptoms, then additional immunosuppression or antifibrotic drugs pirfenidone and/or nintedanib can be considered to treat progressive fibrosing of CPFE.
In conclusion, in advanced NSCLC, PD-1 and programmed death ligand-1 inhibitors are now established treatment options in the first- and second-line settings. However, recent studies have not shown their safety or effect in CPFE-LC patients. We reported two CPFE-LC cases, one case experienced AE-ILD, which was considered an immune-related adverse event, and the history of radiation pneumonitis or AE-ILD maybe increase the risk of AE-ILD in patients with CPFE-LC. Adverse effects may limit the use of immunotherapy in CPFE-LC because of an acute exacerbation of pulmonary fibrosis or conversion to progressive fibrosing phenotype in this challenging clinical situation.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1526/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1526/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ufuk F. Combined Pulmonary Fibrosis and Emphysema. Radiology 2020;296:275. [Crossref] [PubMed]
- Cottin V. The impact of emphysema in pulmonary fibrosis. Eur Respir Rev 2013;22:153-7. [Crossref] [PubMed]
- Li C, Wu W, Chen N, et al. Clinical characteristics and outcomes of lung cancer patients with combined pulmonary fibrosis and emphysema: a systematic review and meta-analysis of 13 studies. J Thorac Dis 2017;9:5322-34. [Crossref] [PubMed]
- Xiaohong X, Liqiang W, Na L, et al. Management and Prognosis of Interstitial Lung Disease With Lung Cancer (ILD-LC): A Real-World Cohort From Three Medical Centers in China. Front Mol Biosci 2021;8:660800. [Crossref] [PubMed]
- Nasim F, Moua T. Lung cancer in combined pulmonary fibrosis and emphysema: a large retrospective cohort analysis. ERJ Open Res 2020;6:e00521-2020. [Crossref] [PubMed]
- Kagohashi K, Satoh H. Successfully managed combined pulmonary fibrosis and emphysema in a lung large cell neuroendocrine carcinoma patient treated with pembrolizumab. Clin Respir J 2021;15:574-5. [Crossref] [PubMed]
- Maeda R, Funasaki A, Motono N, et al. Combined pulmonary fibrosis and emphysema predicts recurrence following surgery in patients with stage I non-small cell lung cancer. Med Oncol 2018;35:31. [Crossref] [PubMed]
- Pepin EW, Verma N, Mehta HJ, et al. Combined Pulmonary Fibrosis and Emphysema. Ann Am Thorac Soc 2018;15:110-2. [Crossref] [PubMed]
- Cottin V. Combined pulmonary fibrosis and emphysema: bad and ugly all the same? Eur Respir J 2017;50:1700846. [Crossref] [PubMed]
- Choi SH, Lee HY, Lee KS, et al. The value of CT for disease detection and prognosis determination in combined pulmonary fibrosis and emphysema (CPFE). PLoS One 2014;9:e107476. [Crossref] [PubMed]
- Kenmotsu H, Yoh K, Mori K, et al. Phase II study of nab-paclitaxel + carboplatin for patients with non-small-cell lung cancer and interstitial lung disease. Cancer Sci 2019;110:3738-45. [Crossref] [PubMed]
- Ichihara E, Miyahara N, Maeda Y, et al. Managing Lung Cancer with Comorbid Interstitial Pneumonia. Intern Med 2020;59:163-7. [Crossref] [PubMed]
- Ogura T, Takigawa N, Tomii K, et al. Summary of the Japanese Respiratory Society statement for the treatment of lung cancer with comorbid interstitial pneumonia. Respir Investig 2019;57:512-33. [Crossref] [PubMed]
- Naccache JM, Gibiot Q, Monnet I, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018;10:3829-44. [Crossref] [PubMed]
- Adashek JJ, Kato S, Ferrara R, et al. Hyperprogression and Immune Checkpoint Inhibitors: Hype or Progress? Oncologist 2020;25:94-8. [Crossref] [PubMed]
- Yamagata A, Yokoyama T, Fukuda Y, et al. Impact of interstitial lung disease associated with immune checkpoint inhibitors on prognosis in patients with non-small-cell lung cancer. Cancer Chemother Pharmacol 2021;87:251-8. [Crossref] [PubMed]
- Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 2017;50:1700050. [Crossref] [PubMed]
- Teng F, Li M, Yu J. Radiation recall pneumonitis induced by PD-1/PD-L1 blockades: mechanisms and therapeutic implications. BMC Med 2020;18:275. [Crossref] [PubMed]


