
miR-137 represses migration and cell motility by targeting COX-2 in non-small cell lung cancer
Introduction
Lung cancer is one of the most common malignant tumors globally, and has become the leading cause of death from malignant tumors in the urban Chinese population (1). According to the pathological characteristics and differentiation degree of cancer cells, lung cancer can be divided into small cell lung cancer (SCLC) (15%) and non-small cell lung cancer (NSCLC) (85%) (2), the latter which includes squamous cell carcinoma (SCC), adenocarcinoma, and large cell carcinoma. Compared with small cell carcinoma, the growth and division of NSCLC cells are slower, and diffusion and metastasis are relatively late (3). About 75% of NSCLC sufferers are diagnosed at an advanced stage, and the 5-year survival rate is very low (4,5). The pathogenesis of lung cancer is complex and understanding its potential molecular mechanisms is of great significance to improve the survival rate of patients and reduce mortality from the disease (6).
MicroRNA (miRNA) is a kind of non-coding single stranded RNA molecule with a length of about 22 nucleotides encoded by endogenous genes. miRNAs participate in the regulation of post transcriptional gene expression in animals and plants (7,8), and are both important regulatory factors in tumors and closely related to the occurrence and development of lung cancer (9,10). After abnormal expression, miRNA can be used as carcinogenic miRNA or tumor suppressor miRNA to regulate the expression of signal pathway genes and affect the proliferation, migration, invasion, and metastasis of lung cancer cells (9,11).
More than 50% of human miRNAs are in special chromosomal regions, which are amplified, deleted, or translocated during tumor development. miRNAs play an important role in the occurrence and evolution of tumors (12) by regulating the physiological processes of cells such as apoptosis, cell proliferation, cell cycle control, DNA repair, and metabolism, and negatively regulating the expression of genes and proteins as carcinogens or tumor suppressors (9,13,14). miR-137 has been proven to hold a tumor suppressor gene function, and its antitumor effect has been confirmed in a variety of cancers (15,16). In our previous study, its promoter methylation was associated with NSCLC cell migration and prognosis (17). And, the A549 cells were treated with cigarette smoking extract for 16 weeks, miR-137 was one of the miRNAs with significantly different expression. In this study we investigated the expression of miR-137 in NSCLC tissues and cells and its effect on the migration and invasion of NSCLC cells and related mechanisms. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2177/rc).
Methods
Patients and tissues
We obtained the paraffin embedded cancerous and paracancerous tissues of 10 NSCLC patients (four with squamous cell cancer and six with adenocarcinoma) who attended the Subei People’s Hospital in 2012. The tissues of ten patients with benign lung lesions were also collected. To further assay the relationship between miRNA expression and the clinicopathological features of NSCLC, a further 56 NSCLC cancerous tissues embedded with paraffin collected between 2008 to 2009 were also obtained. No patient had received chemotherapy before surgical excision, and the resected tissues were embedded with paraffin after being fixed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research was approved by the Ethics Committee of the Subei People’s Hospital of Jiangsu Province, and written informed consent was obtained from all patients prior to participation.
RNA isolation and real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted and purified from paraffin embedded tissues using an RNeasy FFPE Kit (Univ-Bio Company, Shanghai, China), with Trizol applied to isolate total RNA from cultured cells. cDNA was synthesized using a miRcute miRNA cDNA synthesis kit (Tiangen, Biotech, Beijing, China). Primers were synthesized by the Shenggong Company (Shanghai, China), and their sequences are listed in Table 1. SYBR Green and specific primers of miR-137 and U6 were employed to detect the expression of miR-137, and U6 was applied as internal reference.
Table 1
| Gene | Primer sequence (5’-3’) |
|---|---|
| miR-137 | Forward: CAAGGCTTGTTAACACTGTAAC |
| Reverse: TCTGTCAATGTCTGAATAAATG | |
| U6 | Forward: CTCGCTTCGGCAGCACATATACT |
| Reverse: ACGCTTCACGAATTTGCGTGTC |
RT-qPCR, real-time quantitative polymerase chain reaction.
Transwell assay
A transwell chamber with or without matrigel was employed to investigate the effect of miR-137 on the migration or invasion of NSCLC cells. Briefly, the H1299 or A549 cells were digested and subcultured into the upper chamber with or without matrigel after 24 hours of transfection with miR-137 mimic or inhibitor. After a further 24 hours, a cotton swab was adopted to wipe out the cells not passing through polycarbonate membranes, while those passing through pore membranes were fixed with 4% paraformaldehyde and stained with Giemsa for 20 min. The cells were observed with a Leica microscope and analyzed by ImageJ.
Cell culture and transfection
BEAS-2B (Human bronchial epithelial cells), NCI-H1299 cells, and A549 cells (human NSCLC cell line) were obtained from Procell Life Science & Technology Co., Ltd. (Wuhan, China), and were cultured in a 37 ℃ and 5% CO2 constant-temperature incubator with RPMI-1640 medium containing 10% fetal calf serum (HyClone, ThermoFisher, Shanghai). Every second day, the cells were digested with typsin and subcultured, and during their logarithmic growth period, were transfected according to the following protocol.
The miR-137 mimic, mimic negative control, miR-137 inhibitor, inhibitor negative control, COX-2 siRNA, and scramble siRNA were synthesized by RiboBio Co., Ltd (Guangzhou, China). The coding sequence of COX-2 was cloned into lentivirus vector GV492 to obtain the recombined overexpression vector Lv-COX-2, which was provided by Vigen Biotechnology (Zhengjiang, China). The mimic, inhibitor, or siRNA was transfected into H1299 or A549 cells with lipofectamine 2000 (Invitrogen, Shanghai, China). In brief, the cells were digested with typsin and cultured into a 6-well plate with 50,000 cells/well before 24 hours of transfection. The miR-137 mimic, inhibitor (50 pmol), or COX-2 siRNA (100 pmol) were diluted in OPTI-MEM and transfected into NSCLC cells, and Lv-COX-2 was transfected into A549 cells at a multiplicity of infection (MOI) of 5.
Luciferase reporter gene assay
The online software miRanda (http://www.microrna.org/microrna/getMirnaForm.do) was employed to analyze the target genes of miR-137, and as a predictive result, COX-2 (PTGS2) was a target gene of miR-137. The 3’-UTR of COX-2 with or without miR-137 binding mutation site was cloned into pGL3 vector, and the constructed recombined vector pGL3-PTGS2 (WT) or pGL3-PTGS2 (MT) was co-transfected with miR-137 mimic into the logarithmic growth phase of HEK293 cells. After 48 hours transfection, the luciferase was detected using a Luciferase Assay system (Promega Biotech, Beijing, China).
Western blot
The NSCLC H1299 or A549 cells were collected after 72 hours transfection with miR-137 mimic, COX-2 siRNA, or Lv-COX-2, respectively. RIPA lysis solution was applied to extract total protein, and a BCA assay kit was used to quantify its concentration. Total proteins with 50 µg/lane were separated by SDS-PAGE electrophoresis, then transblotted into PVDF membrane. After blocking, the membrane was incubated with rabbit anti-human primary antibodies, COX-2 (1:2,000) (BioVision, Waltham, MA), vimentin (1:2,000) (Merck, Rockville, MD), E-cadherin (1:5,000) (Abcam, Waltham, MA), and β-actin (1:5,000) (Beyotime Biotechnology, Haimen, China), overnight at 4 ℃, respectively. Membranes were then reacted with goat HRP-conjugated anti-rabbit secondary antibody (sigma, St. Louis, MO) for 1 hour at 37 ℃ following washing with TBST. The bands were visualized by enhanced chemiluminescence (ECL) reagent using a Tanon 5200 image system (Tanon, Shanghai, China) and quantified using ImageJ.
Statistical analysis
All data are presented as mean ± standard deviation (SD). Student’s t two-tailed tests were employed to perform comparison between two groups, and one-way ANOVA with post hoc Holm-Sidak correction was performed for multiple comparisons. GraphPad Prism 8 was employed to analyze the data, Kaplan-Meier and log-rank tests were adopted to analyze the disease-free survival and overall survival effected by miR-137. P<0.05 was determined as statistically significant.
Results
Expression of miR-137 in NSCLC tissues and cells
We used RT-qPCR to investigate the expression of miR-137 in NSCLC tissues and cells, with BEAS-2B, a human normal bronchial epithelial cells, as control. The results showed the expression of miR-137 was dramatically down-regulated in NSCLC cell lines (H1299 and A549), compared to BEAS-2B (P<0.01) (Figure 1), and as anticipated, was markedly down-regulated in cancerous tissues compared with paracancerous normal tissues and benign lung tissues (P<0.01) (Figure 1B). The expression analysis of miR-137 in ten pairs of cancerous tissues and paracancerous normal tissues is shown in Figure 1C.
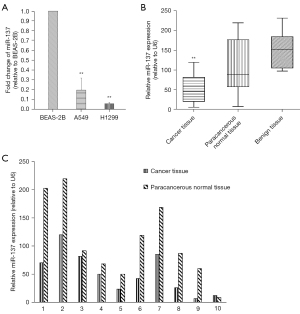
Relationship between miR-137 expression and clinicopathological characteristics of NSCLC
As stated above, the expression of miR-137 was down-regulated in NSCLC tissues and cells, and we further investigated this relationship in 56 NSCLC cancerous tissues. The results showed the expression of miR-137 was correlated with smoking history, lymph node metastasis, and TNM clinical stage (P=0.032, P=0.01 and P=0.015, respectively), while there was no apparent relationship with gender, age, histological type of tumor, tumor size, or tumor differentiation (P>0.05) (Table 2).
Table 2
| Characteristics | miR-137 expression levels | Pa | ||
|---|---|---|---|---|
| Expression value of miR-137 | Low, N | High, N | ||
| Gender | 0.388 | |||
| Male | 329.3±241.0 | 21 | 22 | |
| female | 265.4±195.5 | 7 | 6 | |
| Age, years | 0.362 | |||
| <60 | 327.1±251.7 | 13 | 11 | |
| ≥60 | 305.0±218.2 | 15 | 17 | |
| Smoking status | 0.032 | |||
| 0 | 412.5±262.6 | 7 | 11 | |
| <1 pack/day | 340.1±252.4 | 6 | 7 | |
| ≥1 pack/day | 230.4±163.9 | 15 | 10 | |
| Histological type | 0.288 | |||
| Adenocarcinoma | 344.2±252.3 | 15 | 16 | |
| Squamous cell | 277.6±200.9 | 13 | 12 | |
| T-status | 0.053 | |||
| T1 | 383.2±241.4 | 11 | 19 | |
| T2 | 239.3±200.3 | 14 | 7 | |
| T3 | 217.4±187.0 | 3 | 2 | |
| T4 | – | 0 | 0 | |
| N-status | 0.010 | |||
| N0 | 378.0±222.7 | 12 | 19 | |
| N1 | 312.4±265.3 | 7 | 7 | |
| N2 | 137.8±83.0 | 9 | 2 | |
| N3 | - | 0 | 0 | |
| TNM | 0.015 | |||
| I | 399.6±230.8 | 9 | 17 | |
| II | 285.0±202.2 | 9 | 8 | |
| III | 182.5±209.0 | 10 | 3 | |
| IV | - | 0 | 0 | |
| Differentiation | 0.272 | |||
| Well | 415.9±220.2 | 4 | 7 | |
| Moderate | 290.2±218.2 | 18 | 16 | |
| Poor | 288.0±272.9 | 6 | 5 | |
a, P values for one way analysis of variance (ANOVA) tests. Data were presented as mean ± SD. NSCLC, non-small cell lung cancer; T-status, tumor status; N-status, nodes status; TNM, tumor-nodes-metastases; SD, standard deviation.
Effect of miR-137 on migration and invasion in NSCLC cells
To investigate the effect of miR-137 on the migration and invasion of NSCLC cells, the synthesized miR-137 mimic and inhibitor were subjected to transfection. After 48 hours transfection, the analytical results of RT-qPCR showed miR-137 expression was substantially increased or decreased in NSCLC cells transfected with miR-137 mimic or inhibitor (both P<0.01), respectively, compared to the negative control. The synthesized miR-137 mimic and inhibitor were then adopted for application in the following experiments.
Metastasis of various organs can occur in the late stage of lung cancer, which often brings great suffering to patients and threatens their lives. Transwell assay was employed to detect the influence of migration or invasion by miR-137 and showed its overexpression by mimic markedly restrained the migration or invasion of A549 and H1299 cells. Conversely, miR-137 expression down-regulated by inhibitor significantly facilitated migration or invasion. These results are shown in Figure 2.
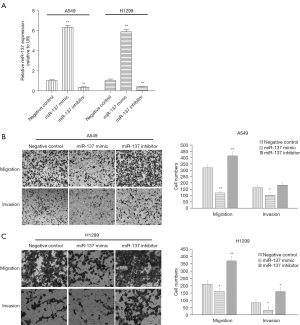
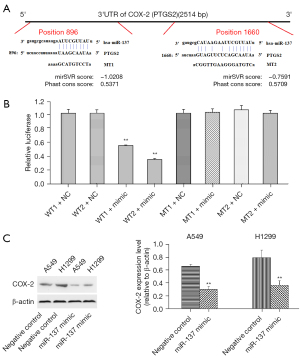
Target gene of miR-137
miRNAs play important roles in multiple life processes, including tumorigenesis and progress, by regulating target genes. As predicted, COX-2 (PTGS2) was a target gene of miR-137, and there were binding sites in the 3’-UTR of COX-2, as shown in Figure 3A. The luciferase reporter gene system was adopted to verify COX-2 as the target gene of miR-137, and showed relative luciferase activity dramatically declined in cells co-transfected with miR-137 mimic and pGL3-PTGS2 (WT). This result directly validated COX-2 as the target gene of miR-137 (Figure 3B). The effects of miR-137 on COX-2 were then detected by Western blot and showed COX-2 expression was apparently suppressed in H1299 and A549 cells transfected with miR-137 mimic (Figure 3C).
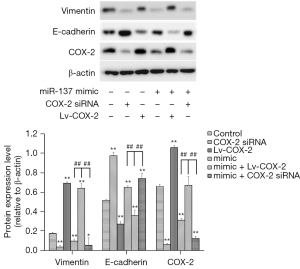
Mechanism of inhibition of migration and invasion by miR-137 in NSCLC cells
Epithelial-Mesenchymal Transition (EMT) plays a crucial role in embryonic development, tissue reconstruction, and cancer metastasis (18), and whether miR-137 suppressed the migration and invasion by regulating EMT was then examined. The expression influences of E-cadherin and vimentin by miR-137 were measured by Western blot, and showed the expression of vimentin was dramatically downregulated in A549 cells alone transfected with miR-137 mimic, while that of E-cadherin was dramatically upregulated. In addition, when A549 cells were co-transfected with Lv-COX-2 and miR-137 mimic, the expression of vimentin was significantly enhanced, and E-cadherin was reduced (Figure 4).
Association between miR-137 expression and survival of NSCLC patients
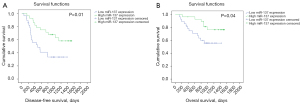
Among the recruited 56 patients with NSCLC, one died of postoperative pneumonia, and the follow-up data of eight patients were lost. A Kaplan-Meier survival curve was applied to analyze the correlation between miR-137 expression and survival in the remaining 47 patients, who were divided based on the relative expression of miR137 into two groups. A high expression group containing 23 patients with miR-137 expression ≥ median and a low expression group containing 24 patients with miR-137 expression < median were established, and as shown in Figure 5, those with high miR-137 expression had apparent longer disease-free survival (P=0.01) and overall survival (P=0.04).
Discussion
miRNAs may act as tumor suppressors or carcinogens in lung cancer by targeting and controlling the expression of multiple signal pathway genes and affecting the proliferation, migration, invasion, and other malignant processes of tumor cells (19,20). In recent years, with the deepening of research, miRNAs have increasingly been recognized as lung cancer biomarkers, and the regulatory mechanism of lung cancer-related miRNAs in tumors has been found (21,22). However, the self-regulation mechanism of miRNA is unclear, and whether this affects the occurrence and evolution of tumors requires further research and exploration (23,24). At the same time, although the mechanism of miRNA in the occurrence and development of lung cancer has been widely studied, its precise mechanism in regulating the malignant biological process of lung cancer cells has not been clarified. miRNA has broad clinical application prospects, and is expected to become a key target for molecular targeted therapy of lung cancer (19,25). Studying the potential molecular mechanisms involved is of great significance for the diagnosis, treatment, and prognosis of a variety of malignant tumors, including lung cancer.
In this study, miR-137 expression was dramatically down-regulated in NSCLC tissues and cells, and the results indicated it may be a tumor suppressor of the disease. Further research showed miR-137 suppressed the migration and invasion of NSCLC cells, and its expression correlated with the smoking history, lymph node metastasis, and TNM clinical stage of NSCLC patients.
Prostaglandin E (PGE) plays an important role in human physiological regulation, involving multiple processes such as inflammation, coagulation, cell growth, tumorigenesis, and development (26,27). Cyclooxygenase (COX), also known as prostaglandin peroxidase, is the rate-limiting enzyme in the process of PGE synthesis, and can metabolize arachidonic acid (AA) to form various prostaglandin (PG) products (28). The human body contains two different COX isoenzyme isomers: Constitutive COX-1 and inducible COX-2. While COX-2 is minimally expressed in normal tissue, this can increase as much as 80 fold, and large amounts can be expressed under the stimulation of inflammatory factors, such as IL-1, TNF-α, lipopolysaccharide (LPS), and cAMP, (29), promoting the synthesis of many PGEs and triggering an inflammatory reaction (30). PGEs produced by COX-2 have a variety of biological activities and can participate in pathophysiological processes through a variety of pathways including tumor cell growth, metastasis, chemoresistance, and tumor stem cell proliferation (31,32). Therefore, the up regulation of COX-2 expression is considered closely related to inflammation, pain, tumorigenesis, and cancer development.
COX-2 is highly expressed in gastric cancer, esophageal adenocarcinoma, colorectal cancer, and other gastrointestinal tumors, and can promote tumorigenesis and tumor development by regulating the expression of genes related to tumor cell proliferation and apoptosis (33). Bothe specific and non-specific inhibitors of COX-2, such as nonsteroidal anti-inflammatory drugs (NSAIDs), can induce apoptosis of gastric cancer, colorectal cancer, and pancreatic cancer cells (34). A study on esophageal cancer showed celecoxib could inhibit the expression of COX-2, down regulate the expression of PGE, inhibit the proliferation of esophageal cancer cells, and improve the therapeutic effect of chemotherapy and radiotherapy (35).
The biological role of miRNA is to regulate target genes, and in this study, online software and luciferase reporter gene systems were employed to predict and validate COX-2 as a target gene of miR-137, which observably suppressed it. COX-2 has an oncogene function in lung cancer, and we identified miR-137 as playing a tumor suppressor role by regulating it.
EMT has been widely studied in malignant tumors and is considered to play a crucial role in chemotherapy tolerance and the migration and invasion of cancer cells (18,36). While during EMT, the expression of epithelial markers (E-cadherin) decreased, interstitial markers (N-cadherin and vimentin) increased in cancer tissues (36,37). EMT is observed in the invasion and metastasis of many malignant tumors, including NSCLC (38). In this study, miR-137 suppressed the migration and invasion of NSCLC cells by inhibiting vimentin and promoting E-cadherin.
In summary, the expression of miR-137 was significantly down-regulated in NSCLC tissues and cells. miR-137 suppressed the migration and invasion of NSCLC cells through regulating EMT relative proteins by targeting COX-2, and correlated with the progress of the disease. It is expected to become a novel biomarker and therapeutic target of NSCLC in the future.
Acknowledgments
The authors are most grateful to all participants in this study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2177/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2177/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2177/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The current study was approved by the Institutional Ethics Committee of the Subei People’s Hospital of Jiangsu Province. The participants gave informed consent before taking part.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu F, Wang L, Zhou C. Lung cancer in China: current and prospect. Curr Opin Oncol 2021;33:40-6. [Crossref] [PubMed]
- Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med 2020;41:1-24. [Crossref] [PubMed]
- Alexander M, Kim SY, Cheng H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020;198:897-907. [Crossref] [PubMed]
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit Rev Oncol Hematol 2021;157:103194. [Crossref] [PubMed]
- Sankar K, Gadgeel SM, Qin A. Molecular therapeutic targets in non-small cell lung cancer. Expert Rev Anticancer Ther 2020;20:647-61. [Crossref] [PubMed]
- Van Meter EN, Onyango JA, Teske KA. A review of currently identified small molecule modulators of microRNA function. Eur J Med Chem 2020;188:112008. [Crossref] [PubMed]
- Mahjoubin-Tehran M, Rezaei S, Jalili A, et al. A comprehensive review of online resources for microRNA-diseases associations: the state of the art. Brief Bioinform 2022;23:bbab381. [Crossref] [PubMed]
- Ali Syeda Z, Langden SSS, Munkhzul C, et al. Regulatory Mechanism of MicroRNA Expression in Cancer. Int J Mol Sci 2020;21:1723. [Crossref] [PubMed]
- Akram F, Atique N, Haq IU, et al. MicroRNA, a Promising Biomarker for Breast and Ovarian Cancer: A Review. Curr Protein Pept Sci 2021;22:599-619. [Crossref] [PubMed]
- Hussen BM, Hidayat HJ, Salihi A, et al. MicroRNA: A signature for cancer progression. Biomed Pharmacother 2021;138:111528. [Crossref] [PubMed]
- Manso J, Censi S, Mian C. Epigenetic in medullary thyroid cancer: the role of microRNA in tumorigenesis and prognosis. Curr Opin Oncol 2021;33:9-15. [Crossref] [PubMed]
- Taefehshokr S, Taefehshokr N, Hemmat N, et al. The pivotal role of MicroRNAs in glucose metabolism in cancer. Pathol Res Pract 2021;217:153314. [Crossref] [PubMed]
- Szatkowska M, Krupa R. Regulation of DNA Damage Response and Homologous Recombination Repair by microRNA in Human Cells Exposed to Ionizing Radiation. Cancers (Basel) 2020;12:1838. [Crossref] [PubMed]
- Feng Q, Wu Q, Liu X, et al. MicroRNA-137 acts as a tumor suppressor in osteosarcoma by targeting enhancer of zeste homolog 2. Exp Ther Med 2021;22:1168. [Crossref] [PubMed]
- Chen W, Du J, Li X, et al. microRNA-137 downregulates MCL1 in ovarian cancer cells and mediates cisplatin-induced apoptosis. Pharmacogenomics 2020;21:195-207. [Crossref] [PubMed]
- Min L, Wang F, Hu S, et al. Aberrant microRNA-137 promoter methylation is associated with lymph node metastasis and poor clinical outcomes in non-small cell lung cancer. Oncol Lett 2018;15:7744-50. [Crossref] [PubMed]
- Zhang N, Ng AS, Cai S, et al. Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol 2021;22:e358-68. [Crossref] [PubMed]
- Zhong S, Golpon H, Zardo P, et al. miRNAs in lung cancer. A systematic review identifies predictive and prognostic miRNA candidates for precision medicine in lung cancer. Transl Res 2021;230:164-96. [Crossref] [PubMed]
- Iqbal MA, Arora S, Prakasam G, et al. MicroRNA in lung cancer: role, mechanisms, pathways and therapeutic relevance. Mol Aspects Med 2019;70:3-20. [Crossref] [PubMed]
- Hu C, Meiners S, Lukas C, et al. Role of exosomal microRNAs in lung cancer biology and clinical applications. Cell Prolif 2020;53:e12828. [Crossref] [PubMed]
- Cheng Y, Yang S, Shen B, et al. Molecular characterization of lung cancer: A two-miRNA prognostic signature based on cancer stem-like cells related genes. J Cell Biochem 2020;121:2889-900. [Crossref] [PubMed]
- Hill M, Tran N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech 2021;14:dmm047662. [Crossref] [PubMed]
- Hill M, Tran N. MicroRNAs Regulating MicroRNAs in Cancer. Trends Cancer 2018;4:465-8. [Crossref] [PubMed]
- Amiri A, Pourhanifeh MH, Mirzaei HR, et al. Exosomes and Lung Cancer: Roles in Pathophysiology, Diagnosis and Therapeutic Applications. Curr Med Chem 2021;28:308-28. [Crossref] [PubMed]
- Ye Y, Wang X, Jeschke U, et al. COX-2-PGE2-EPs in gynecological cancers. Arch Gynecol Obstet 2020;301:1365-75. [Crossref] [PubMed]
- Wilson DJ, DuBois RN. Role of Prostaglandin E2 in the Progression of Gastrointestinal Cancer. Cancer Prev Res (Phila) 2022;15:355-63. [Crossref] [PubMed]
- Lai ZZ, Yang HL, Ha SY, et al. Cyclooxygenase-2 in Endometriosis. Int J Biol Sci 2019;15:2783-97. [Crossref] [PubMed]
- Hashemi Goradel N, Najafi M, Salehi E, et al. Cyclooxygenase-2 in cancer: A review. J Cell Physiol 2019;234:5683-99. [Crossref] [PubMed]
- Davis FM, Tsoi LC, Wasikowski R, et al. Epigenetic regulation of the PGE2 pathway modulates macrophage phenotype in normal and pathologic wound repair. JCI Insight 2020;5:138443. [Crossref] [PubMed]
- Cen B, Lang JD, Du Y, et al. Prostaglandin E2 Induces miR675-5p to Promote Colorectal Tumor Metastasis via Modulation of p53 Expression. Gastroenterology 2020;158:971-984.e10. [Crossref] [PubMed]
- Wang D, Fu L, Sun H, et al. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology 2015;149:1884-1895.e4. [Crossref] [PubMed]
- Gong Z, Huang W, Wang B, et al. Interplay between cyclooxygenase-2 and microRNAs in cancer Mol Med Rep 2021;23:347. (Review). [Crossref] [PubMed]
- Sheng J, Sun H, Yu FB, et al. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int J Med Sci 2020;17:1095-101. [Crossref] [PubMed]
- Yin X, Zhang Y, Wen Y, et al. Celecoxib alleviates zinc deficiency-promoted colon tumorigenesis through suppressing inflammation. Aging (Albany NY) 2021;13:8320-34. [Crossref] [PubMed]
- Lee HW, Jose CC, Cuddapah S. Epithelial-mesenchymal transition: Insights into nickel-induced lung diseases. Semin Cancer Biol 2021;76:99-109. [Crossref] [PubMed]
- Cai J, Cui Y, Yang J, et al. Epithelial-mesenchymal transition: When tumor cells meet myeloid-derived suppressor cells. Biochim Biophys Acta Rev Cancer 2021;1876:188564. [Crossref] [PubMed]
- Bronte G, Puccetti M, Crinò L, et al. Epithelial-to-mesenchymal transition and EGFR status in NSCLC: the role of vimentin expression. Ann Oncol 2019;30:339-40. [Crossref] [PubMed]


