
Long-term survival of a non-small cell lung cancer patient with EGFR-mutated brain metastases: a case report
Introduction
Lung cancer is one of the most common cancers worldwide. A significant number of patients have advanced disease at the time of diagnosis. Targeted therapy plays an important role in the treatment of advanced lung cancer; epidermal growth factor receptor (EGFR)-mutations predict the response of EGFR-tyrosine kinase inhibitors (TKIs) such as gefitinib, erlotinib, afatinib, or dacomitinib, with response rates of around 70% (1). T790M mutation accounts for approximately half of the acquired resistance to EGFR-TKI (2). Osimertinib has been developed to act against T790M resistance mutation. Brain metastases (BMs) cause morbidity and mortality in patients with non-small cell lung cancer (NSCLC) (3). It was reported that 10% to 20% of NSCLC patients have BMs at their first diagnosis, and 25% to 50% of NSCLC patients will ultimately develop BMs during the course of the disease (4). The median and 1-, 2-, and 3-year overall survival (OS) for NSCLC patients with BMs were 6 months and 29.9%, 14.3%, and 8.4% respectively (5). BMs remain a tricky problem in NSCLC patients and impose a distinct challenge for clinicians. Several therapeutic schedules are efficacious for these patients which include third-generation EGFR-TKIs, EGFR-TKIs + anti-vascular endothelial growth factor receptor (VEGFR), EGFR-TKIs + stereotactic radiosurgery/whole brain radiotherapy (SRS/WBRT) and second-generation EGFR-TKI (6). Yet preferable treatments for EGFR-mutated NSCLC with BMs are elusive. Our case shares an NSCLC patient who achieved an OS over 8 years after brain metastasis through a range of therapies but without chemotherapy. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1671/rc).
Case presentation
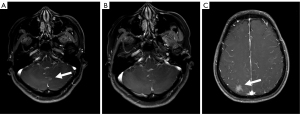
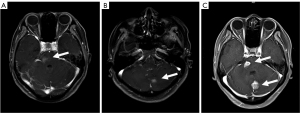
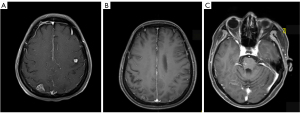
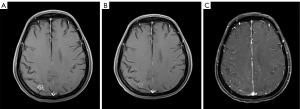
A 53-year-old woman, never smoked, was diagnosed with EGFR-mutant (exon 19 deletion, diagnosis method: ADx-ARMs) NSCLC with asymptomatic solitary brain metastasis on March 15, 2014, merely 1 month after lung cancer surgery (Figure 1A). No brain metastasis was detected preoperatively by enhanced magnetic resonance imaging (MRI). The patient was postoperatively diagnosed as left upper lung adenosquamous carcinoma (predominant in adenocarcinoma component) with interlobar lymph node metastasis [American Joint Committee on Cancer (AJCC) version 7 staging pT2aN1M0]. Once diagnosed with brain metastasis, the patient initiated gefitinib treatment (250 mg daily), and then the size of brain metastasis shrinked significantly 1 month after treatment (Figure 1B). Gefitinib was initially well-tolerated with confirmation of disease response for 40 months, then MRI documented disease progression in the brain showing new lesions appeared in the right parietal lobe and right pons and the original lesion in left cerebellar hemisphere enlarged (Figures 1C,2A,2B). As no tumor site was suitable for biopsy while disease progression was merely limited to the brain, a digital polymerase chain reaction (PCR) assay for EGFR T790M mutation on plasma sample was performed and revealed a negative result. Chemotherapy and a cerebral spinal fluid (CSF) gene detection assay were recommended but both were refused by the patient. Considering no extracranial relapse, the patient was given erlotinib (150 mg/day) and whole brain radiotherapy (30 Gy in 10 fractions) followed by boosted dose to the gross tumor (10 Gy in 5 fractions for pons tumor and 15 Gy in 5 fractions for the rest of the metastatic tumor). Two months later, the patient developed serious neurologic symptoms of numbness in the left upper limb and brain edema, and a brain MRI documented disease progression demonstrating a new metastatic lesion in the left frontal lobe and enlargement of the previously irradiated brain lesions (Figures 2C,3A). Meanwhile a bone scan revealed new metastasis in the sternum. A repeated plasma liquid biopsy showed the same T790M negative state as before. The off-label use for osimertinib was admitted and she was switched to osimertinib treatment (80 mg/day) on December 14, 2017. The patient then achieved disease control quickly, with her neurologic symptoms disappeared (Figure 3B,3C). The patient was diagnosed with the progression of brain disease with a newly developed brain mass after 22 months of osimertinib treatment (Figure 4A). Since then, she initiated bevacizumab (7.5 mg/kg i.v. every 21 day) without osimertinib interruption. The newly developed brain mass resolved after 1 month of treatment (Figure 4B,4C) and the disease is still responding until the last follow-up (May 2022). During the course, a next generation sequencing (NGS) assay for 23 NSCLC cancer-related genes on plasma samples was performed again on April 25, 2022 and revealed the same EGFR-mutant (exon 19 deletion).
In the case report, treatment consent was obtained from the patient, and she is satisfied with the therapeutic schedule and the results so far. We have de-identified the details such that the identity of the patient may not be ascertained in any way. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In our case, the NSCLC patient with EGFR-mutated brain metastasis achieved long-term survival. The OS is more than 8 years and the progression-free survival (PFS) of gefitinib was 40 months, whereas the PFS of osimertinib monotherapy was 22 months, and the combination therapy of osimertinib and bevacizumab has a PFS of 31 months and has not progressed so far.
A prospective phase II study in Japan evaluated the efficacy of gefitinib alone without radiation therapy for the treatment of patients with brain metastasis from lung adenocarcinoma, the result showed an objective response rate (ORR) of 87.8%, a median PFS of 14.5 months (95% CI: 10.2–18.3 months) and a median OS of 21.9 months (95% CI: 18.5–30.3 months) (7). Compared with L858R, exon 19 deletion was associated with a better outcome for patients after treatment with gefitinib in both PFS (10.2 vs. 17.5 months, P=0.003) and OS (19.8 vs. 30.3 months, P=0.025) (7). Another prospective clinical phase II study in Korea revealed that NSCLC patients harboring EGFR mutation with brain metastasis and receiving gefitinib or erlotinib achieved a disease control rate (DCR) of 93%, and the median PFS and OS were 6.6 months (95% CI: 3.8–9.3 months) and 15.9 months (95% CI: 7.2–24.6 months), respectively (8). In our case, the PFS of gefitinib was 40 months, which is significantly longer than the literature reported (14.5 months) (7).
In this case, when the disease progressed after gefitinib treatment, the lesions were intracranial, and the patient refused CSF biopsy. A liquid biopsy on plasma sample revealed negative for T790M mutation, thus there was no indication for the 3rd generation TKI. Under such circumstances, chemotherapy can be used after gefitinib treatment. However, the progression after gefitinib treatment was limited intracranial. A related study reported that after the failure of advanced NSCLC with gefitinib, erlotinib can achieve a good effect with 5–10% partial response (PR) and 40–60% stable disease (SD) (9), which could be explained by erlotinib’s higher biological concentration and better permeability of the blood-brain barrier. Considering the synergetic efficacy of EGFR-TKIs + SRS/WBRT in EGFR-mutant NSCLC with brain metastasis (6), erlotinib was used in combination with brain radiotherapy to control brain metastasis. But it didn’t work in our case.
Osimertinib had favorable blood-brain barrier permeability and showed significant efficacy in brain metastasis (10). In the phase 3 FLAURA study, the median PFS of advanced NSCLC patients with EGFR-mutation and brain metastasis having received osimertinib (as first-line treatment) was 15.2 months (95% CI: 12.1–21.4 months), the ORR was 66% (11), among them, the T790M status were unknown. In the phase 1 AURA1-trial, 61 patients without T790M mutation achieved an ORR of 21%, DCR of 61% and a median PFS of 2.8 months (95% CI: 2.1–4.3 months) (12). The TREM-study is a multi-center, phase 2 clinical trial conducted in five Northern European countries, in the T790M-negative cohort treated with osimertinib after disease progression on at least one previous EGFR-TKI, patients had a response rate of 28% and PFS of 5.1 months (95% CI: 2.6–7.6 months), within the T790M negative group, the ORR for patients with BMs and without was 33% vs. 26% (P=0.718) (1). The AURA1-trial was a dose expansion trial, 20 patients received daily doses lower than the recommended 80 mg, that might explain the lower efficacy compared to the latter study (12). Our results were in agreement with the real-world study which the application of osimertinib further improves survival outcomes regardless of T790M status (3).
In our case, after treatment with osimertinib, the PFS was 22 months, the survival time was significantly prolonged and clinical symptoms were improved. The mechanism for this observed activity of osimertinib in patients without T790M-mutation remains unclear, but might at least in part due to a false negative T790M situation caused by too low circulatory tumor DNA (ctDNA) concentration in peripheral blood to be detected; The mechanism of T790M resistance might be squamous cell transformation, pleomorphic transformation, and EGFR mutations (L718Q and C797S) (13). Other explanations suggested that there was difference in the timing of emergence of T790M mutation; some investigators demonstrated that the T790M status in an individual patient can be spatiotemporally and temporally heterogeneous due to selective pressure from EGFR-TKI (2). In our case, T790M mutation was negative by peripheral blood. Though the reliability of liquid biopsy of T790M on plasma sample is poor when the tumor burden is low, it is difficult to obtain a biopsy of tumor tissue when the disease is merely limited to the brain and the patient refused CSF biopsy, we chose liquid biopsy. The reported sensitivity of plasma genotyping for the detection of T790M was 70% and the false-negative rate was 30% (14), so we repeated blood biopsy to reduce the false-negative rate. During the treatment course, we performed a total of four genetic tests, and the technologies used in liquid biopsy include droplet digital PCR and NGS. Tissue biopsy is the most accurate detection method to determine the status of T790M, but it involves numerous challenges in terms of availability, safety, and cost.
Both osimertinib and bevacizumab can cross the blood-brain barrier, and have comparable effectiveness in the central nervous system (CNS) (15). Combination treatment of osimertinib and bevacizumab may work against BMs. Yu et al. present a phase 1/2 study of bevacizumab and osimertinib as initial treatment for patients with EGFR mutant lung cancers with BMs and 49 patients were included, the results suggest that combination treatment may be efficacious and protective against CNS progression in which the CNS response rate was 100%, the ORR was 80%, the median PFS was 19 months (13). In our case, T790M mutation was not detected in the patient, osimertinib as the third line TKI achieved a long-term response before intracranial disease progression. At the time of the third intracranial disease progression, bevacizumab was added to osimertinib as a fourth-line treatment and achieved nearly complete response of the newly developed metastasis and long-term control of BMs with no overlapping toxic effects.
Overall, the patient in our case achieved a long-term survival with the OS over 8 years after brain metastasis through a range of therapies but without chemotherapy. Baek et al. reported that the overall median survival after BM development (BM-OS) was significantly longer in patients with EGFR mutation type (MT) than in those with EGFR wild type (WT) (25.7 vs. 3.8 months, P<0.001), and in patients with metachronous BM, BM-OS in patients with EGFR mutations was 14.5 months (16). Lung-mol Graded Prognostic Assessment (GPA) is recommended as an index to prognosticate patients with BM from NSCLC. According to Lung-mol GPA, younger age, better performance status, absence of extracranial metastasis (ECM), a smaller number of BM, and the EGFR mutation or anaplastic lymphoma kinase (ALK) rearrangement predict a favorable survival (17). Patients with more CNS lesions had inferior outcomes, and the number of involved extracranial organs was an independent prognostic factor for NSCLC patients with BM. In patients with single-organ ECM, a better prognosis was observed in lung and bone metastasis, while liver metastasis showed the worst survival (4).
Conclusions
Our case showed that EGFR-TKI (1st and 3rd generation TKI) alone is effective in the control of brain metastasis in NSCLC. Osimertinib can be tried after 1st generation TKI resistance even if with no EGFR T790M detected by liquid biopsy, bevacizumab added to osimertinib is effective for disease control when the intracranial disease progressed with osimertinib alone. Our case also denotes that radiation therapy for patients with asymptomatic brain metastasis could be delayed for a better quality of life in NSCLC patients. Osimertinib has been recognized as an effective treatment for BMs, and osimertinib in combination with bevacizumab may benefit as an upfront treatment for NSCLC with EGFR mutation. The novelty of our case is prolonged use of osimertinib in combination with the 4th line bevacizumab that achieved a longer survival. However, the benefit of bevacizumab in patients with intracranial progression after osimertinib therapy is not universal. This is a case report and further studies are needed to demonstrate which patients may benefit from combination therapy. In conclusion, our case shares a patient with EGFR-mutated BMs accepting a series of treatments but without chemotherapy and resulting in significantly prolonged survival with an OS over 8 years and improved clinical symptoms; it is promising to achieve a long-term survival in advanced NSCLC with multiple brain metastasis as well as systemic progression.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1671/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1671/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eide IJZ, Helland Å, Ekman S, et al. Osimertinib in T790M-positive and -negative patients with EGFR-mutated advanced non-small cell lung cancer (the TREM-study). Lung Cancer 2020;143:27-35. [Crossref] [PubMed]
- Hata A, Katakami N, Yoshioka H, et al. Spatiotemporal T790M Heterogeneity in Individual Patients with EGFR-Mutant Non-Small-Cell Lung Cancer after Acquired Resistance to EGFR-TKI. J Thorac Oncol 2015;10:1553-9. [Crossref] [PubMed]
- Yu X, Sheng J, Pan G, et al. Real-world utilization of EGFR TKIs and prognostic factors for survival in EGFR-mutated non-small cell lung cancer patients with brain metastases. Int J Cancer 2021;149:1121-8. [Crossref] [PubMed]
- Wang M, Wu Q, Zhang J, et al. Prognostic impacts of extracranial metastasis on non-small cell lung cancer with brain metastasis: A retrospective study based on surveillance, epidemiology, and end results database. Cancer Med 2021;10:471-82. [Crossref] [PubMed]
- Waqar SN, Samson PP, Robinson CG, et al. Non-small-cell Lung Cancer With Brain Metastasis at Presentation. Clin Lung Cancer 2018;19:e373-9. [Crossref] [PubMed]
- Zhao B, Wang Y, Wang Y, et al. Efficacy and safety of therapies for EGFR-mutant non-small cell lung cancer with brain metastasis: an evidence-based Bayesian network pooled study of multivariable survival analyses. Aging (Albany NY) 2020;12:14244-70. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556-60. [Crossref] [PubMed]
- Singh N, Jindal A, Behera D. Erlotinib usage after prior treatment with gefitinib in advanced non-small cell lung cancer: A clinical perspective and review of published literature. World J Clin Oncol 2014;5:858-64. [Crossref] [PubMed]
- Nanjo S, Ebi H, Arai S, et al. High efficacy of third generation EGFR inhibitor AZD9291 in a leptomeningeal carcinomatosis model with EGFR-mutant lung cancer cells. Oncotarget 2016;7:3847-56. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Yu HA, Schoenfeld AJ, Makhnin A, et al. Effect of Osimertinib and Bevacizumab on Progression-Free Survival for Patients With Metastatic EGFR-Mutant Lung Cancers: A Phase 1/2 Single-Group Open-Label Trial. JAMA Oncol 2020;6:1048-54. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Offin M, Feldman D, Ni A, et al. Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers. Cancer 2019;125:4380-7. [Crossref] [PubMed]
- Baek MY, Ahn HK, Park KR, et al. Epidermal growth factor receptor mutation and pattern of brain metastasis in patients with non-small cell lung cancer. Korean J Intern Med 2018;33:168-75. [Crossref] [PubMed]
- Sperduto PW, Yang TJ, Beal K, et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol 2017;3:827-31. [Crossref] [PubMed]


