
The role of bronchoscopy as a novel approach in preoperative lung marking for early-stage lung cancer
Introduction
We read the recently published article, “Comparison of bronchial methylene blue staining and modified inflation-deflation method in identifying the intersegmental plane during lung segmentectomy,” by Wang and colleagues with great interest (1). We congratulate the authors on this successful submission on an emerging topic and appreciate the opportunity to offer a few comments.
Lung cancer is the second most common cancer and leading cause of cancer-related deaths in the United States. In 2021, the United States Preventative Services Task Force (USPSTF) modified the lung cancer screening guideline to include ages 50–80 (previously 55–80) and reduced pack years of smoking from 30 to 20 (2). With these updated guidelines, there is an expected increase of 6.4 million more eligible patients compared to the 2013 recommendations (3). The updated guidelines may increase the number of patients that are diagnosed with early-stage lung cancer who are likely to benefit the most from sublobar resections with curative intent. The most recent National Comprehensive Cancer Network (NCCN) guidelines for stage 1A non-small cell lung cancer (NSCLC) recommends sublobar resection, defined as anatomical segmentectomy (preferred) or wedge resection, in select patient populations.
As discussed in the original article, there is literature available comparing sublobar (segmentectomy) versus lobectomy in patients with stage 1A NSCLC with studies showing no difference in overall survival (OS) rate (94% vs. 91%). Additionally, the 5-year relapse free survival was not different between the two groups (88% vs. 87.9%) demonstrating no difference in locoregional recurrence (4). Similarly, there has been no notable difference in 30- and 90-day mortality between sub-lobar and lobar resection. The 5-year OS was 64.3% in the lobectomy compared to 63.8% in the sublobar group. The outcomes of sublobar resection were non-inferior to lobectomy in patients with stage 1A lesions (5). These studies suggest that employing a lung preservative approach with sublobar resection is non-inferior to lobectomy and a feasible approach in the management of early-stage lung cancer. These data suggest that further precision is required to help map out the area before operative intervention using either dye marking or fiducial placement. This can be done either via a CT-guided transthoracic or bronchoscopic approach.
In this editorial, we describe the various methods available via a bronchoscopic approach to mark target lesions or segments to assist in surgical interventions. The advent of newer technologies, such as electromagnetic navigation bronchoscopy (ENB) and robotic-assisted bronchoscopy (RAB), have helped a bronchoscopist to navigate farther safely and accurately into the lung periphery. In addition, the use of augmented fluoroscopy (AF), cone beam computed tomography (CBCT), and C-arm based tomography (CABT) has increased the accuracy to localize lesions with real-time feedback. Studies have demonstrated that combined RAB, radial endobronchial ultrasound (r-EBUS), and fluoroscopy use leads to 96% localization of lesions (6). With this added precision, and these platforms are widely used for diagnostic purposes, we anticipate they will have an increased utility in assisting with preoperative planning, and operative interventions. The transition to minimally invasive techniques, e.g., video-assisted thoracoscopic surgery (VATS) and robot-assisted thoracoscopic surgery (RATS), may limit the operator’s ability to manually palpate small lesions, part-solid and ground glass nodules. However, with the use of ENB or RAB combined with real-time feedback from r-EBUS, CBCT, or CABT, bronchoscopy has an adjunctive role to accurately localize the lesion. Furthermore, use of fiducial markers (FMs), pleural dye marking, and virtual assisted lung mapping (VAL-MAP) are potential technologies to use via bronchoscopy to aid surgeons in the management of small peripheral nodules.
FMs
FMs are radiographically opaque tools that are positioned within or adjacent to a target lesion, typically a tumor, to help localize a lesion for stereotactic body radiation (SBRT) or surgical resection. These markers may be placed via a percutaneous route by interventional radiologists or bronchoscopically. Multiple prior studies have demonstrated the feasibility and accuracy of bronchoscopically placed FMs for both pre-operative surgical planning and stereotactic radiation therapy (7-9). Belanger and colleagues performed FM placement in 65 patients with a total of 133 markers placed. 99% of the markers were confirmed durable on imaging or resected lung specimen (7). Similarly, Bowling and colleagues performed a subgroup analysis of NAVIGATE study where a total of 563 FMs were placed in 258 patients with a pneumothorax rate of 5% (9). These studies suggest that bronchoscopy is a feasible approach in marking the lesions or adjacent area with accuracy and can be done at the same time as the diagnostic procedure to help the surgeons define their approach better intraoperatively. An example of FM placement can be seen in Figure 1.

Pleural dye marking
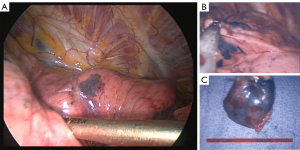
Pleural dye marking is typically performed preoperatively to allow for visualization of the anatomical planes. The two most common agents utilized are methylene blue and indigo carmine. Additionally, indocyanine green (ICG) can be used for fluorescent marking. Typically, 0.5 to 1.0 mL of agent are instilled into three separate locations to allow appropriate triangulation of the surgical plane. Bolton and colleagues reported the use of intraoperative ENB with methylene blue pleural dye marking in 19 patients with subsequent resection in 16 patients. All patients that underwent resection were noted to have successful localization (10). More recently, Jeong and colleagues published on ENB guided transbronchial dye-marking and VATS as a single stage procedure. Eighteen pulmonary nodules in 7 patients were localized using intra-operative ENB indigo carmine placement. Dye placement was successfully performed in 94% (11). A subgroup analysis of the NAVIGATE study with 23 patients who underwent dye marking prior to surgery showed adequacy in 91% patients with 95% of the lesions located in the peripheral 1/3rd of the lung. The most common dye used was methylene blue in 91% cases. The average procedure time was 22 minutes with an average ENB procedure time of 11.5 minutes. No complications were noted in the patients (12). Figure 2 shows an example of a pleural dye marking performed using navigation bronchoscopy.
VAL-MAP
Lastly, VAL-MAP is a novel technology that combines pleural dye marking and lesion marking. The latest iteration, VAL-MAP 2.0, utilizes ENB navigation to place multiple pleural dye markers followed by bronchoscopic placement of a coil into or adjacent to the lesion. This creates both an anatomical plane for resection with appropriate margins as well as localization of the lesion of interest. VAL-MAP can be performed pre-operatively as a stand-alone procedure or on the same day. Sato and colleagues published results of a multicenter, prospective single-arm study of VAL-MAP 2.0 in 64 patients, including a total of 65 nodules. Successful sublobar resections was achieved in 98%. Dye marking was successfully located in 88% of the cases. Additionally, 85% of micro-coils were within 5 mm of the target lesion on post-mapping CT. There were 14 unsuccessful placements during the procedures. There were no major adverse events due to VAL-MAP 2.0. Importantly, the authors discussed how VAL-MAP 2.0 can be utilized even in the absence of an bronchus sign (bronchus that terminates at the tumor) (13). VAL-MAP is another example how combining the field of advanced/navigational bronchoscopy with the expertise of video assisted thoracoscopy or robotic thoracoscopy improves the care of patients with low grade NSCLC, which is of paramount importance in an era of rapidly expanding lung cancer screening programs.
Conclusions
Wang and colleagues explored an exciting approach to integrate these goals with the use of methylene blue dye marking to shorten operative time as compared to inflation-deflation technique (1). Similarly, our group would like to highlight the opportunity to employ navigational bronchoscopy to achieve similar goals. FMs, pleural dye mapping, and VAL-MAP allow for more precise resection planes with the recommended margin of 2 centimeters. When compared to more invasive techniques such as transthoracic marking, bronchoscopy allows for fewer surgical complications, specifically with reduced rates of pneumothoraces. Bronchoscopic techniques can also be combined with surgical resection on the same day, another opportunity to limit surgical complications by decreasing total operative time and number of procedures. It is important to consider the role of each field and technology as we progress to new frontiers in peripheral tumor management. We believe the future of peripheral lung lesions management lies in the combination of different modalities—including navigational bronchoscopy guided diagnosis with tumor localization interventions and minimally invasive surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2827/coif). DKH reports that he is a stock option holder/stockholder of Body Vision, Broncus, Eolo, Eon, Gravitas, Imbio, Lanier, Magnisity, Noah Medical, LX-Medical, Med-Opsys, Monogram Orthopedics, Preora, Preview Med, Prothea-X, Ryme, Ruby Robotics, Spesana, and VIDA. He is consultant within last 3 years for Alpha Sights, Ambu, Atheneum, Auris, BioPlusRx, Body Vision, Boston Scientific, Broncus, Coleman, CSL, Deerfield, Eolo, Fluidda, Gala, Gilman Capital, GLG, Grand Rounds, Guidepoint Global, Imbio, InhibRx, J&J, Lanier, Level-Ex, Magnisity, MediFind, Morgan-Stanley, Mosaic, Noah Medical, NovaScan, Olympus (Spiration), Oncocyte, Patients Like Me, Preora, Preview Med, Prothea-X, PulmonX, Qure.ai, Ryme, Ruby Robotics, Serpex, Spesana, Takeda, TSC, Veracyte, Volv, and Wave Life Sciences. He reports Research Dollars/Contracted Research (past 3 years and present) from Gala, Nuvaira, Olympus (Spiration), and PulmonX. He was a DSMB member in the past and was on board of InhibRx. He reports lectures given (honoraria received) within the last 3 years for Astra-Zeneca, Biodesix, B.I., Boston Scientific, Genentech, Grifols, PulmonX, Spiration (Olympus), and Takeda. AW has been a consultant for Noah Medical, Medtronic, and Ambu. He is a Speaker for Biodesix. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang M, Zhang Z, Mei P, et al. Comparison of bronchial methylene blue staining and modified inflation-deflation method in identifying the intersegmental plane during lung segmentectomy. Transl Cancer Res 2022;11:4000-8. [Crossref] [PubMed]
- Krist AH, Davidson KW, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962-70. [Crossref] [PubMed]
- Landy R, Young CD, Skarzynski M, et al. Using Prediction Models to Reduce Persistent Racial and Ethnic Disparities in the Draft 2020 USPSTF Lung Cancer Screening Guidelines. J Natl Cancer Inst 2021;113:1590-4. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest 2021;159:845-52. [Crossref] [PubMed]
- Belanger AR, Burks AC, Chambers DM, et al. Peripheral Lung Nodule Diagnosis and Fiducial Marker Placement Using a Novel Tip-Tracked Electromagnetic Navigation Bronchoscopy System. J Bronchology Interv Pulmonol 2019;26:41-8. [Crossref] [PubMed]
- Bolton WD, Richey J, Ben-Or S, et al. Electromagnetic Navigational Bronchoscopy: A Safe and Effective Method for Fiducial Marker Placement in Lung Cancer Patients. Am Surg 2015;81:659-62. [Crossref] [PubMed]
- Bowling MR, Folch EE, Khandhar SJ, et al. Fiducial marker placement with electromagnetic navigation bronchoscopy: a subgroup analysis of the prospective, multicenter NAVIGATE study. Ther Adv Respir Dis 2019;13:1753466619841234. [Crossref] [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Jeong JH, Park H, Choi CM, et al. Preoperative electromagnetic navigation bronchoscopy-guided one-stage multiple-dye localization for resection of subsolid nodules: A single-center pilot study. Thorac Cancer 2022;13:466-73. [Crossref] [PubMed]
- Folch EE, Bowling MR, Pritchett MA, et al. NAVIGATE 24-Month Results: Electromagnetic Navigation Bronchoscopy for Pulmonary Lesions at 37 Centers in Europe and the United States. J Thorac Oncol 2022;17:519-31. [Crossref] [PubMed]
- Sato M, Nagayama K, Kobayashi M, et al. Virtual-Assisted Lung Mapping 2.0: Preoperative Bronchoscopic Three-Dimensional Lung Mapping. Ann Thorac Surg 2019;108:269-73. [Crossref] [PubMed]


