
Paget disease of the breast with underlying invasive ductal cancer in neurofibromatosis type 1: a case report
Highlight box
Key findings
• We present a rare case of a patient with neurofibromatosis type 1 (NF1) and Paget disease accompanied by invasive breast cancer.
What is known and what is new?
• Patients with NF1 are more likely to develop breast cancer compared to the general population.
• Paget disease of the breast is an unusual observation in patients with NF1 and breast cancer.
What is the implication, and what should change now?
• Early breast cancer screening should be performed for patients with NF1.
• Breast-conserving surgery should be chosen carefully to avoid the risk of carcinogenesis from postoperative radiotherapy.
• Regular follow-up should be performed to detect cancers early in the contralateral breast for those who have already had breast cancer on one side.
Introduction
Neurofibromatoses (NFs) are a group of rare genetic disorders that cause tumors to form on nerve tissue (1). There are 3 NF subtypes: NF1, NF2, and schwannomatosis. NF1, also called Von Recklinghausen disease, accounts for 96% of cases of NF and is inherited from a parent or caused by spontaneous neurofibromin 1 (NF1) gene mutation (1,2). The most common signs and symptoms of NF1 are coffee-colored café-au-lait spots on the skin, Lisch nodules on the iris, and neurofibromas mostly under the skin. The NF1 gene is also a tumor-suppressor gene, and thus NF1 mutations are associated with several malignancies, the most common being intracranial gliomas and malignant peripheral nerve sheath tumors (MPNSTs) (3). Other malignancies, such as breast cancer, pheochromocytoma, gastrointestinal stromal tumors, and malignant fibrous histiocytoma, are also related to NF1 mutations (3).
Paget disease of the breast (PDB) is a rare type of cancer that is characterized by inflammatory eczema-like changes of the nipple (4). PDB is usually associated with an underlying invasive breast cancer or develops alone (5). Many genes responsible for breast cancer have been identified, such as BRCA1 DNA repair associated (BRCA1) and BRCA2 DNA repair associated (BRCA2) (6). Numerous studies have suggested that NF1 mutations may lead to a higher incidence of breast cancer (3,7). However, reports of patients with NF1 and Paget disease accompanied by invasive breast cancer are rare (8).
Both NF1 and breast cancer have the same sign: a lump in the breast. This makes it difficult to detect breast cancer in patients with NF1, thereby delaying treatment. At present, guidelines for the treatment for patients with NF1 and breast cancer are scarce (9), but in many recognized NF1-related cancers, surgical intervention is routinely used. Uusitalo et al. reported that breast cancer subtypes with NF1 were more often associated with estrogen receptor (ER) and progesterone receptor (PR) loss, human epidermal growth factor receptor 2 (HER2) amplification, and a higher tumor grade, all of which are indicators of a poor prognosis (10). We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1783/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 50-year-old woman with a palpable mass in her right breast was admitted to the Second Hospital of Jilin University on March 25, 2019. The patient had been diagnosed with NF1 when she was 16 years old. Her father had NF1, but her mother, brother, and sister did not. Physical examinations revealed a firm and immobile mass in the upper quadrant of the right breast; the skin of the breast showed no edema, redness, or pitting. However, eczematous changes of the nipple and areola were found. The skin all over the patient’s body had large amounts of light-brown (café-au-lait) spots of various sizes and pea-sized bumps (neurofibromas) even on her breasts (Figure 1A,1B). The patient did not feel skin discomfort.
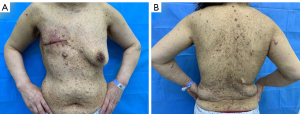
Enlarged axillary and supraclavicular lymph nodes were not palpable on either side.
Ultrasound examinations revealed multiple hypoechoic masses in the upper outer quadrant of the right breast. The biggest mass measured 2.2 cm × 1.0 cm and had an unclear boundary, irregular shape, and spot-like strong echo calcifications inside. The mass was categorized as Breast Imaging-Reporting and Data System (BI-RADS) category 4C (Figure 2). No enlarged lymph nodes were seen in the bilateral axillae. Radiographs detected no metastatic lesions.
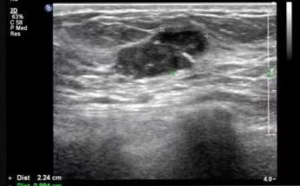
An ultrasound-guided core needle biopsy was performed. Pathology results revealed invasive breast cancer. The patient underwent right breast mastectomy and sentinel lymph node biopsy on March 29, 2019.
Postoperative pathology confirmed the previous diagnosis: invasive ductal carcinoma grade III (1.5 cm in diameter; Figure 3A) without vessel infiltration. Moderate- to high-grade ductal carcinoma in situ (DCIS) was found among the interstitium below the nipple, involving the skin of the nipple (Figure 3B). Paget cells were also observed in the nipple of the right breast (Figure 3C). Sentinel lymph node biopsy revealed there was no metastasis in the right sentinel lymph node (0/3). Immunohistochemical staining results were as follows: ER, 90% with strong intensity; PR, 90% with strong intensity; HER2, 3+; E-cadherin positive; P53 negative; and a Ki67 labeling index of 60% (Figure 3D-3H).
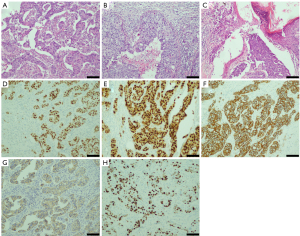
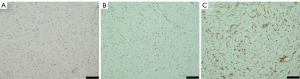
Pathology of the cutaneous neurofibromas under the skin of the breast was also performed. We observed hypocellular proliferation of spindle cells with interspersed collagen bundles, as well as mast cells. Immunohistochemistry revealed that the nodule was S100 positive and CD34 positive (Figure 4).
The final diagnosis was PDB with underlying invasive ductal cancer and NF1. Subsequently, the patient underwent adjuvant chemotherapy with epirubicin and cyclophosphamide every 3 weeks for 4 cycles, followed by docetaxel every 3 weeks for 4 cycles. Trastuzumab was given concomitantly with docetaxel once every 3 weeks for 1 year. When chemotherapy ended, the patient started 5-year endocrine therapy with tamoxifen. As of this writing, the patient has not exhibited any severe side effects and is being followed up. Up to 44 months after surgery (last follow-up November 25, 2022), no local recurrence or metastases have been found by physical and imaging examinations. Figure 5 shows a timeline of the diagnosis, treatment, and follow-up in this patient.

Discussion
According to the diagnostic criteria developed by the National Institutes of Health and revised in 2021 (11), an individual who does not have a parent diagnosed with NF1 and meets at least 2 of the following criteria can be diagnosed with NF1: (I) ≥6 café-au-lait macules with a greatest diameter >5 mm in prepubertal individuals and >15 mm in postpubertal individuals; (II) freckling in the axillary or inguinal region; (III) ≥2 neurofibromas of any type or 1 plexiform neurofibroma; (IV) optic pathway glioma; (V) ≥2 Lisch nodules (iris hamartomas) or ≥2 choroidal abnormalities; (VI) a distinctive osseous lesion, such as sphenoid dysplasia, anterolateral bowing of the tibia, or pseudarthrosis of a long bone; and (VII) a heterozygous pathogenic NF1 variant with a variant allele fraction of 50% in apparently normal tissue, such as white blood cells.
Some individuals with NF1 have barely noticeable neurological problems, whereas others are profoundly affected. The life span of patients with NF1 is significantly shortened (by 16.5 years in men and by 26.1 years in women) compared with the general population primarily because patients with NF1 are likely to have malignant peripheral nerve sheath tumors that are very aggressive (12,13).
Although the incidence of NF1 with breast cancer is extremely low, current data show that the incidence of malignancy in individuals with NF1 is higher than in the general population (14). Uusitalo et al. conducted a cohort study to assess the incidence and mortality of cancer among 1,404 (737 women) Finnish patients with NF1, 31 of whom (4.2%) had breast cancer. They found the breast cancer incidence of NF1 in women aged <40 years to be 10-fold that of the control population (10). A meta-analysis conducted by Suarez-Kelly et al. found that women with NF1 have a 3-fold increased risk of developing breast cancer than did the general population [standardized incidence ratio (SIR) 3.07; 95% confidence interval (CI): 2.16–4.38) (15)]. Compared with the general population, women with NF1 aged <50 years had a 5-fold increased risk of breast cancer (SIR 5.08; 95% CI: 3.77–6.81) (15). Furthermore, breast cancer is the second most frequently diagnosed cancer in patients with MPNST (16). Breast cancer patients with NF1 also have a worse prognosis (10). A retrospective study from Japan showed that these patients were likely to have a younger age at onset and advanced clinical stage, and to be hormone receptor negative (17). These features are indicative of a worse prognosis.
NF1 is an autosomal dominant genetic disorder caused by an NF1 gene germline mutation, with an approximate birth incidence of 1 in 2,500–3,500 individuals; however, half of all patients have new NF1 mutations (18,19). McPherson et al. found independent somatic NF1 mutations in 3 separate tumor specimens from a patient who was diagnosed with breast cancer, MPNST, and neurofibroma, indicating that a "second hit” of NF1 (somatic mutation after a germline mutation) may be important in tumorigenesis (20). The NF1 gene encodes for neurofibromin, which activates RAS-GTPase (21). Breast cancer is considered to be the result of the accumulation of mutations in epithelial cells, and the NF1 gene is considered a tumor-suppressor gene in breast cancer, with mutations in NF1 potentially enhancing the frequency of breast cancer malignant transformation (22). Abnormalities in the NF1 gene may be related to genes classically associated with breast cancer. The BRCA1, erb-b2 receptor tyrosine kinase 2 (ERBB2), P53, and NF1 genes are located in the long arm of chromosome 17. This can potentially explain the relationship between NF1 and breast cancer (23). The tumor in our patient had high ER and PR expression. A previous study showed that frameshift and nonsense mutations in NF1 are associated with a poor prognosis in ER-positive breast cancer patients (24).
Neurofibromas are usually found on or under the skin. They can also be found in the breast (25). Therefore, it is important to distinguish neurogenic tumors from breast cancer in the breast area to avoid delayed detection. Moreover, considering the higher risk of breast cancer morbidity among patients with NF1, healthcare professionals should encourage individuals, especially those in high-risk populations, to perform breast self-examinations and to undergo regular early mammogram screening, which could help with the detection of early breast cancer (26). In addition to women with NF1 having an increased risk of a first primary breast cancer, they have a higher rate of contralateral breast cancer, with a cumulative risk of 26.5% in 20 years (27). Thus, a contralateral mastectomy could be considered a preventive measure.
In our patient, the remarkable finding was that Paget disease developed with an invasive cancer. PDB is a rare type of breast cancer, accounting for only 0.5–5% of all breast cancers (4). PDB has the appearance of eczema within the skin involving the nipple and areola. The hallmark of PDB is Paget cells within the epidermis; these are large cells with a pale cytoplasm, atypical nuclei, and prominent nucleoli. One theory to explain the pathogenesis of the PDB is the “epidermotropic” theory, which proposes that Paget cells are ductal carcinoma cells that have migrated to the epidermis of the nipple Another theory explaining the pathogenesis of PDB is the transformation theory, which proposes that epidermal keratinocytes are transformed into malignant Paget cells (28). However, there is more evidence supporting the epidermotropic theory, as ductal involvement immediately below the nipple and the same or similar immunohistochemical markers as the primary site have been reported (4,29). The main pathological type of these underlying cancers is HER2 positive (4). PDB with underlying invasive cancer or DCIS is commonly seen, but only a few cases of PDB with NF1 have been reported (8,30). Because of its rarity, there is currently insufficient evidence to prove the relationship between PDB and NF1. However, neurofibromas are more likely to develop in the nipple-areolar zone, which is the most common site of PDB (31). Findings from genetic studies may provide clues as to the links among invasive breast cancer, DCIS, PDB, and NF1, and to identifying new therapeutic targets.
Zheng et al. reported that neurofibromin, the product of the NF1 gene, is an ERα transcriptional corepressor (32). NF1 depletion enhanced ER transcription and acquired resistance to endocrine therapy. Mitogen-activated protein kinase kinase (MEK) inhibitors have shown potential in the treatment of NF-deficient ER-positive breast cancer (29). However, there is no clear evidence of the clinical efficacy of MEK inhibitors. Pearson et al. found that NF1 may mutate during metastasis, inducing the resistance to endocrine therapy, and that combined palbociclib and fulvestrant may overcome this resistance (21). In our patient, adjuvant therapy was administered according to the guidelines for breast cancer (33). The association between NF1 and the pathogenesis of breast cancer is obscure, and there are no available drugs targeting NF1. To determine the adequacy of conventional treatments for breast cancer patients with NF1, extensive data validation is required. NF1 may be an important prognostic factor to guide treatment.
Mastectomy may be a better choice for patients with NF1 breast cancer because there is a chance that radiotherapy after breast-conserving surgery may lead to cancer (34). In addition, according to the postoperative pathological classification of breast cancer, systemic treatments, such as chemotherapy, endocrine therapy, and anti-HER2 therapy, should follow existing breast cancer treatment guidelines (32).
In this paper, we report on a case of NF1 and PDB with underlying invasive ductal cancer. Other than our case, only 3 cases of breast cancer accompanied by PDB with NF1 have been reported in the literature (8,30).
Conclusions
Patients with NF1 have a higher risk of developing breast cancer. However, definite proof of the association between NF1 and breast cancer is still required. Key points are as follows: (I) mutations of NF1, as a tumor-suppressor gene, may increase tumor susceptibility, including to breast carcinoma and PDB; (II) patients with NF1, especially those who have numerous neurofibromas in their breast, should undergo early screening for breast cancer to avoid delays in detection; (III) treatment of breast cancer with NF1 should be conducted according to the current breast cancer guidelines, with breast-conserving surgery chosen carefully to avoid the risk of carcinogenesis from postoperative radiotherapy; and (IV) patients who have already had breast cancer on one side should undergo regular follow-up to detect cancers early in the contralateral breast.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1783/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1783/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Farschtschi S, Mautner VF, McLean ACL, et al. The Neurofibromatoses. Dtsch Arztebl Int 2020;117:354-60. [PubMed]
- Kresak JL, Walsh M. Neurofibromatosis: A Review of NF1, NF2, and Schwannomatosis. J Pediatr Genet 2016;5:98-104. [Crossref] [PubMed]
- Uusitalo E, Rantanen M, Kallionpää RA, et al. Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J Clin Oncol 2016;34:1978-86. [Crossref] [PubMed]
- Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget's disease of the nipple. Breast Cancer Res Treat 2013;141:1-12. [Crossref] [PubMed]
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer 2006;107:1448-58. [Crossref] [PubMed]
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017;317:2402-16. [Crossref] [PubMed]
- Madanikia SA, Bergner A, Ye X, et al. Increased risk of breast cancer in women with NF1. Am J Med Genet A 2012;158A:3056-60. [Crossref] [PubMed]
- Kawawa Y, Okamoto Y, Oharaseki T, et al. Paget's disease of the breast in a woman with neurofibromatosis. Clin Imaging 2007;31:127-30. [Crossref] [PubMed]
- Stewart DR, Korf BR, Nathanson KL, et al. Care of adults with neurofibromatosis type 1: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2018;20:671-82. [Crossref] [PubMed]
- Uusitalo E, Kallionpää RA, Kurki S, et al. Breast cancer in neurofibromatosis type 1: overrepresentation of unfavourable prognostic factors. Br J Cancer 2017;116:211-7. [Crossref] [PubMed]
- Legius E, Messiaen L, Wolkenstein P, et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: an international consensus recommendation. Genet Med 2021;23:1506-13. [Crossref] [PubMed]
- Uusitalo E, Leppävirta J, Koffert A, et al. Incidence and mortality of neurofibromatosis: a total population study in Finland. J Invest Dermatol 2015;135:904-6. [Crossref] [PubMed]
- Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 2002;39:311-4. [Crossref] [PubMed]
- Walker L, Thompson D, Easton D, et al. A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br J Cancer 2006;95:233-8. [Crossref] [PubMed]
- Suarez-Kelly LP, Yu L, Kline D, et al. Increased breast cancer risk in women with neurofibromatosis type 1: a meta-analysis and systematic review of the literature. Hered Cancer Clin Pract 2019;17:12. [Crossref] [PubMed]
- Williams LA, Moertel CL, Richardson M, et al. Incidence of second malignancies in individuals diagnosed with malignant peripheral nerve sheath tumors. J Neurooncol 2020;147:701-9. [Crossref] [PubMed]
- Yamagishi Y, Einama T, Yamasaki T, et al. Metachronous bilateral triple-negative breast cancer associated with neurofibromatosis type 1: A case report. Oncol Lett 2019;17:2818-24. [Crossref] [PubMed]
- Blakeley JO, Plotkin SR. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol 2016;18:624-38. [Crossref] [PubMed]
- Griffiths S, Thompson P, Frayling I, et al. Molecular diagnosis of neurofibromatosis type 1: 2 years experience. Fam Cancer 2007;6:21-34. [Crossref] [PubMed]
- McPherson JR, Ong CK, Ng CC, et al. Whole-exome sequencing of breast cancer, malignant peripheral nerve sheath tumor and neurofibroma from a patient with neurofibromatosis type 1. Cancer Med 2015;4:1871-8. [Crossref] [PubMed]
- Pearson A, Proszek P, Pascual J, et al. Inactivating NF1 Mutations Are Enriched in Advanced Breast Cancer and Contribute to Endocrine Therapy Resistance. Clin Cancer Res 2020;26:608-22. [Crossref] [PubMed]
- Dekkers JF, Whittle JR, Vaillant F, et al. Modeling Breast Cancer Using CRISPR-Cas9-Mediated Engineering of Human Breast Organoids. J Natl Cancer Inst 2020;112:540-4. [Crossref] [PubMed]
- Yap YS, McPherson JR, Ong CK, et al. The NF1 gene revisited - from bench to bedside. Oncotarget 2014;5:5873-92. [Crossref] [PubMed]
- Griffith OL, Spies NC, Anurag M, et al. The prognostic effects of somatic mutations in ER-positive breast cancer. Nat Commun 2018;9:3476. [Crossref] [PubMed]
- Woo OH, Yong HS, Lee JB, et al. A giant malignant peripheral nerve sheath tumour of the breast: CT and pathological findings. Br J Radiol 2007;80:e44-7. [Crossref] [PubMed]
- Charu V, Cimino-Mathews A. Peripheral nerve sheath tumors of the breast. Semin Diagn Pathol 2017;34:420-6. [Crossref] [PubMed]
- Evans DGR, Kallionpää RA, Clementi M, et al. Breast cancer in neurofibromatosis 1: survival and risk of contralateral breast cancer in a five country cohort study. Genet Med 2020;22:398-406. [Crossref] [PubMed]
- Karakas C. Paget's disease of the breast. J Carcinog 2011;10:31. [Crossref] [PubMed]
- Sek P, Zawrocki A, Biernat W, et al. HER2 molecular subtype is a dominant subtype of mammary Paget's cells. An immunohistochemical study. Histopathology 2010;57:564-71. [Crossref] [PubMed]
- Holloway KB, Ramos-Caro FA, Flowers FP. Paget's disease of the breast in a man with neurofibromatosis. Int J Dermatol 1997;36:609-11. [Crossref] [PubMed]
- Bongiorno MR, Doukaki S, Aricò M. Neurofibromatosis of the nipple-areolar area: a case series. J Med Case Rep 2010;4:22. [Crossref] [PubMed]
- Zheng ZY, Anurag M, Lei JT, et al. Neurofibromin Is an Estrogen Receptor-alpha Transcriptional Co-repressor in Breast Cancer. Cancer Cell 2020;37:387-402.e7. [Crossref] [PubMed]
- NCCN. Breast cancer (version 1.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed April 15, 2019.
- Johnson M, Cook L, Rapisarda F, et al. Surgical dilemma of the management of breast cancer in a patient with neurofibromatosis: case report and a review of the literature. J Surg Case Rep 2020;2020:rjaa365. [Crossref] [PubMed]


