
Effectiveness of tislelizumab when combined with etoposide and carboplatin in patients with SMARCA4-deficient undifferentiated thoracic tumor: a case report
Highlight box
Key findings
• The case of SMARCA4-UT with a high mutation burden successfully responded to the combined regimen containing tislelizumab, etoposide, and carboplatin.
What is known and what is new?
• Here is known: presently, no clear guidelines are available for the treatment of SMARCA4-UT;
• Here is new: etoposide and carboplatin combined with tislelizumab were used as the second-line treatment and exhibited good therapeutic effects.
What is the implication, and what should change now?
• It means that gene sequencing and PD-L1 expression should be performed after the diagnosis of all advanced SMARCA4-UTs. For tumors that respond poorly to radiotherapy and chemotherapy (including SMARCA4-UTs), immunotherapy can be tried if there is no immunotherapy hyper-progression gene and there is the existence of possible markers to evaluate the benefit from immunotherapy.
Introduction
SMARCA4 encodes the catalytic subunit of switch/sucrose nonfermentable (SWI/SNF) chromatin remodeling complex, which has ATPase and helicase activities. It involves double-strand break, cell cycle, and cytoskeleton and chromosome organization (1). SMARCA4-deficient undifferentiated thoracic tumor (SMARCA4-UT) is a rare malignancy that is more likely to occur in men and heavy smokers (more than 20 pack-years). SMARCA4-UT often has complete loss or diffuse dilution of Brahma related gene 1 (BRG1) expression. But INI-1 expression is retained. Most cases also lose SMARCA2 expression. Sex-determining region of Y chromosome (SRY)-box2 (SOX2) expression is relatively seen in more than 90% of cases. Cluster of differentiation 34 (CD34) and Spalt like transcription factor 4 (SALL4) expressions are seen in 60% to 70% of cases (2). The tumor cells do not lose mismatch repair (MMR) proteins (3). SMARCA4 is the most often inactivated subunit in the SWI/SNF complex, with a mutation rate of about 0% to 10% in lung cancer patients. TP53, KRAS, STK11, and KEAP1 mutation were found in 30% to 60% of SMARCA4-UTs. The median overall survival (OS) was only four to seven months (4). Several patients are diagnosed in advanced stages of the malignancy and do not respond to conventional radiotherapy and chemotherapy (5,6). Presently, there is no standard treatment for SMARCA4-UTs. However, some recent studies have shown that patients with SMARCA4-UT thoracic tumors benefitted from immunotherapy (7-10), and the longest OS time was up to 22 months. Tislelizumab is a programmed cell death 1 (PD-1) antibody approved for the treatment of non-small cell lung cancer (NSCLC). Here in, we report a case of SMARCA4-UT that successfully responded to the treatment containing tislelizumab, etoposide, and carboplatin (TEC). A combination of immunotherapy and chemotherapy may be a new treatment regimen for patients with SMARCA4-UTs. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1679/rc).
Case presentation
A 51-year-old Chinese male presented to our hospital with the symptom of dry cough. He was a current smoker with a history of 54 pack-years. The patient did not have a chronic history of hypertension or diabetes and any family history of malignant tumors. A chest computed tomography (CT) revealed a 74 mm × 58 mm mass of the right upper mediastinal lymphadenopathy (Figure 1). A CT-guided percutaneous lung biopsy of the lung mass was performed. Subsequent clinicopathological examination confirmed the diagnosis of SMARCA4-UT (Figure 2) and the clinical stage was T4N2M0 stage IIIB. The expressions of nine genes (ALK, ROS1, RET, EGFR, KRAS, NRAS, HER2, PIK3CA, and MET) related to lung cancer were negative by real-time quantitative polymerase chain reaction (PCR). The neoadjuvant therapy was recommended initially and then the surgical intervention was evaluated. The patient was treated with four cycles of liposomal paclitaxel (135 mg/m2 on day 1) and cisplatin (80 mg/m2 on day 1) combined with two cycles of anlotinib (12 mg on days 1 to 14) as the first-line therapy. A CT scan after four courses of induction therapy demonstrated the disease progression. The tumor mass was increased by 30% (92 mm × 96 mm) from the pretreatment size according to the analysis by RECIST version 1.1 (10). The patient experienced severe right chest tightness and thoracalgia. On immunohistochemical analysis, PD-L1 expression was not found. Next-generation sequencing using the Burning Rock OncoScreen Cancer Research Panel revealed a high tumor mutation burden (TMB) at 15.95 mutations/Mb. TMB is defined as the number of somatic mutations per Megabase of the interrogated genomic sequence. A SMARCA4 mutation was detected at the site of c.1105G>T in exon 6 with TP53 missense mutation (c.469G>T in exon 5) and a KEAP1 frameshift mutation (p.T418fs in exon 4). The patient was treated with tislelizumab (200 mg on day 1 and then every 3 weeks), etoposide (70 mg/m2 on days 1 to 3), and carboplatin (350 mg/m2 on day 1) as a second-line treatment due to high TMB. After one TEC administration, the patient’s chest tightness and thoracalgia were improved, suggesting a possible clinical benefit. Strikingly, a partial response (PR) was seen on a chest CT scan (Figure 1C) (11). A chest CT scan after five cycles of TEC demonstrated a sustained PR response but with secondary myelosuppression (platelet count of 62×109/L) (Figure 1D). Subsequently, tislelizumab was administered as the maintenance therapy. Disease-free survival (DFS) has been maintained for more than ten months since the beginning of TEC treatment. The clinicopathologic characteristics of this SMARCA4-UT patient and previous cases are summarized in Table 1.
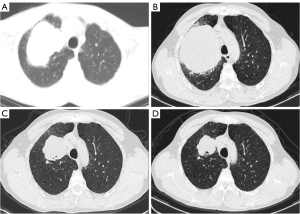
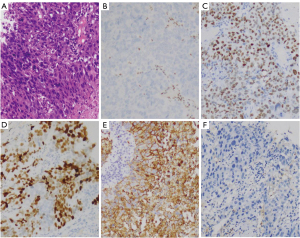
Table 1
| Case number | Age (years) | Sex | Smoking status | Tumor marker | PD-L1 expression | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 60 | Male | Smoker (SI 40) | SOX2, CD34, SALL4, SYN and P53 were positive | NA | NA | Died soon after biopsy | (2) |
| Cases 2–31 | Range, 28–90 (median: 48) | Male:female =9:1 | Smoker | EMA (n=29/30); SOX2 (n=26/27); CD34 (n=17/27); SMARCB1 (23/23); SALL4 (n=7/21) and Claudin-4 (2/19) | NA | Treatment strategies were varied | Median overall survival was 6 months | (5) |
| Case 32 | 72 | Male | Smoker (SI 80) | Tumor marker tests were negative | Negative | Combine atezolizumab, carboplatin, and nab-paclitaxel | Displayed 7 months of stable disease | (6) |
| Case 33 | 73 | Female | Smoker (SI 53) | TMB was 11/Mb, a mutation in SMARCA4 was detected | PD-L1 TPS of 40% | Treated with ABCP treatment as the first-line chemotherapy. After three cycles and switched to the maintenance phase (continued atezolizumab and bevacizumab) | No disease progression and the treatment is still continued after 17 months of initiating treatment | (8) |
| Case 34 | 59 | Male | Smoker (SI 39) | TMB was 11.8/Mb, a mutation in SMARCA4 was detected | Negative | The patient was administered ABCP treatment. After three cycles and switched to the maintenance phase (continued atezolizumab and bevacizumab) | Disease progressed with bilateral adrenal gland and abdominal lymph node metastases after 10 months | (8) |
| Case 35 | 64 | Female | Smoker (SI 44) | TMB was 14.9/Mb, a mutation in SMARCA4 was detected | PD-L1 TPS of 80% | ABCP therapy was administered as the first-line treatment. After three cycles and switched to the maintenance phase (continued atezolizumab and bevacizumab) | After 2 months of maintenance therapy, no disease progression | (8) |
| Case 36 | 70 | Female | NA | NA | Positive | Pembrolizumab | Suppressed tumor growth dramatically, with only one dose leading to a partial response | (9) |
| Case 37 | 58 | Female | NA | NA | Negative | Pembrolizumab | Patient had progressive disease after 12 Gy | (10) |
| Case 38 | 51 | Male | Smoker (SI 54) | TMB was 15.95/Mb with TP53 and KEAP1 mutations | Negative | Combine tislelizumab, etoposide, and carboplatin | A reduction in tumor burden was seen for more than ten months | This paper |
NA, not available; SI, smoking index; TPS, tumor proportion score; TMB, tumor mutation burden; ABCP, atezolizumab in combination with bevacizumab, paclitaxel and carboplatin; PD-L1,programmed cell death ligand 1; SMARCA4-UT, SMARCA4-deficient undifferentiated thoracic tumor.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
SMARCA4-UT is a type of NSCLC with large atypia and low differentiation. It is highly malignant and has a short survival time. There is no standard treatment at present. It is classified under the other lung epithelial tumors of thoracic tumors in the 2021 edition by the World Health Organization (WHO) (12). In early cases, it can be surgically resected, but in late or advanced cases, it relies on radio chemotherapy although it does not respond well (5,13). Presently, the treatment of advanced NSCLC is recommended (14).
The first-line therapy with two cycles of chemotherapy failed in this case. The tumor size increased, and the disease progressed significantly. The first line of chemotherapy combined with anti-tumor angiogenesis treatment also failed, which was consistent with the previous reports (5,15). In recent years, a small number of reports have shown that immunotherapy benefitted SMARCA4-UT patients (8,16). Studies also found that most SMARCA4-UTs had negative PD-L1 expression but exhibited high TMB. Among the major drivers of NSCLC, the loss of either KEAP1 or STK11-induced TMB (17,18). Since somatic mutations generate neoantigens, high TMB is expected to induce an anti-tumor response. However, it can serve as a biomarker predicting favorable responses to immune checkpoint blockades (ICB) (19-21). The prediction of high TMB on the efficacy of immunotherapy was not affected by the expression of PD-L1. Due to such reason, some patients benefitted from immunotherapy and had significantly prolonged survival (16,22,23).
However, poor effects of immunotherapy and severe progression were also reported (24). Some studies revealed that the majority of patients with advanced NSCLC exhibited primary resistance to ICB (20,25). The tumor microenvironment (TME) is a mixture of tumor cells and endogenous host stroma that influence cancer growth and development. The TME can influence the disease prognosis and antitumor immunity. Furthermore, it can influence the outcome and effectiveness of cancer management therapies. Gantzer et al. identified nine SMARCA4-UT patients and eight patients had no tertiary lymphoid structures, consistent with an immune desert TME phenotype (26). In this case, next-generation sequencing was performed and immunotherapy combined with chemotherapy was selected. After this treatment regimen, the tumor size became smaller and the symptoms were relieved, suggesting effective treatment.
SMARCA4-UT does not express PD-L1 but exhibits high TMB and is often accompanied by co-mutations of TP53, KRAS, KEAP1, and STK11. Dong et al. found that patients with KRAS and TP53 mutations benefitted from immunotherapy (27). STK11 is a negative immune regulatory gene. KEAP1 gene is controversial in predicting the efficacy of immunotherapy (28). The co-mutation of STK11 and KEAP1 genes result in a poor prognosis with immunotherapy in lung cancers with high TMB. This outcome may cause poor response and severe progression in some SMARCA4-UTs after using immune checkpoint inhibitors.
About 23% of lung adenocarcinoma and 34% of lung squamous cell carcinoma exhibited KEAP1 mutation and the patients with KEAP1 gene mutation were often heavy smokers, which were characterized by over expression of PD-L1 and high TMB (defined as more than10 mutations/Mb). However, the median OS time was not improved after the use of immunotherapeutic drugs due to less T cell infiltration (cold tumor) (29,30). But the results of some studies were controversial. A survey on patients harboring KEAP1 mutation in different types of cancers in China found that the mutation rate of KEAP1 mutation was the highest in lung squamous cell carcinoma (about 29%) and the survival time of patients harboring mutation treated with immunotherapy was significantly improved compared with other therapies (median OS: 22.52 vs. 12.89 months, P=0.0034) (31). Checkmate227 study showed that patients with KEAP1 mutation had significantly higher PFS (11.1 months) than those with KEAP1 wild type (5.5 months) (32). The keynote-042 study showed that regardless of the mutation status of KEAP1, pablizumab was more effective than platinum-containing chemotherapies (33).
To elucidate the effect of KEAP1 and TP53 gene co-mutation on the prognosis of patients, Professor Saleh et al. conducted a retrospective study to analyze the clinical and gene mutation spectrum data in 6,297 patients with NSCLC. The analysis found that 51.5% (3,245/6,297) patients had TP53 mutation, of which 17.3% (524/3,022) patients had KEAP1 gene co-mutation. Compared with patients with wild-type TP53/KEAP1 or other types of TP53 mutation, patients with TP53 truncation mutation (including frameshift mutation and nonsense mutation) or KEAP1 mutation had significantly shorter OS and DFS, which were negative independent prognostic factors. However, the coexistence of KEAP1 mutation with other types of TP53 (including missense/synonymous/in-frame mutation) brought survival benefits. The mutant TP53 might have played an important role in modifying the KEAP1-NRF2 signaling pathway (34). The coexistence of KEAP1 and TP53 missense mutations in this patient may have been another main reason for the objective remission and survival benefits of the use of immune checkpoint inhibitors.
Conclusions
SMARCA4-UT is a rare malignant condition with high invasiveness and poor prognosis. It does not respond to chemoradiotherapy. In this case, the first-line treatment containing paclitaxel combined with cisplatin and anlotinib was ineffective. Therefore, etoposide and carboplatin combined with tislelizumab were used as the second-line treatment and exhibited good therapeutic effects. Immunotherapy may be a new treatment option for patients with SMARCA4-UTs and when combined with chemotherapy may provide a better effect. This case did not express PD-L1 but expressed KEAP1 mutation. Several studies found that patients with KEAP1 mutation had a poor therapeutic effect with immune checkpoint inhibitors (29,30). However, this patient with high TMB and TP53 missense mutation benefitted from the treatment regimen. A single index is not an ideal biomarker for predicting the efficacy of immunotherapy. For tumors that respond poorly to radiotherapy and chemotherapy (including SMARCA4-UTs), immunotherapy can be tried if there is no immunotherapy hyper-progression gene and there is the existence of possible markers to evaluate the benefit from immunotherapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1679/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1679/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1679/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnaud O, Le Loarer F, Tirode F. BAFfling pathologies: Alterations of BAF complexes in cancer. Cancer Lett 2018;419:266-79. [Crossref] [PubMed]
- Nambirajan A, Singh V, Bhardwaj N, et al. SMARCA4/BRG1-Deficient Non-Small Cell Lung Carcinomas: A Case Series and Review of the Literature. Arch Pathol Lab Med 2021;145:90-8. [Crossref] [PubMed]
- Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol 2017;30:797-809. [Crossref] [PubMed]
- Yoshida A, Boland JM, Loarer FL, et al. Thoracic SMARCA4-deficient undifferentiated tumor. WHO Classification of Tumours Editorial Board. Thoracic Tumours. WHO classification of tumours series, 5th edition; vol. 5. Lyon, France: International Agency for Research on Cancer; 2021. [cited 2021 May 27].
- Perret R, Chalabreysse L, Watson S, et al. SMARCA4-deficient Thoracic Sarcomas: Clinicopathologic Study of 30 Cases With an Emphasis on Their Nosology and Differential Diagnoses. Am J Surg Pathol 2019;43:455-65. [Crossref] [PubMed]
- Utsumi T, Taniguchi Y, Noda Y, et al. SMARCA4-deficient undifferentiated tumor that responded to chemotherapy in combination with immune checkpoint inhibitors: A case report. Thorac Cancer 2022;13:2264-6. [Crossref] [PubMed]
- Nambirajan A, Dutta R, Malik PS, et al. Cytology of SMARCA4-Deficient Thoracic Neoplasms: Comparative Analysis of SMARCA4-Deficient Non-Small Cell Lung Carcinomas and SMARCA4-Deficient Thoracic Sarcomas. Acta Cytol 2021;65:67-74. [Crossref] [PubMed]
- Kawachi H, Kunimasa K, Kukita Y, et al. Atezolizumab with bevacizumab, paclitaxel and carboplatin was effective for patients with SMARCA4-deficient thoracic sarcoma. Immunotherapy 2021;13:799-806. [Crossref] [PubMed]
- Takada K, Sugita S, Murase K, et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: A case report. Thorac Cancer 2019;10:2312-5. [Crossref] [PubMed]
- Henon C, Blay JY, Massard C, et al. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann Oncol 2019;30:1401-3. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO classification of tumours: Thoracic Tumours. 5th ed. Lyon: IARC Press, 2021.
- Zheng MJ, Zheng Q, Wang Y, et al. SMARCA4-deficient primary thoracic sarcoma:a clinicopathological analysis of five cases. Zhonghua Bing Li Xue Za Zhi 2019;48:537-42. [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Hanna NH, Temin S, Masters G. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update Summary. JCO Oncol Pract 2020;16:e844-8. [Crossref] [PubMed]
- Schoenfeld AJ, Bandlamudi C, Lavery JA, et al. The Genomic Landscape of SMARCA4 Alterations and Associations with Outcomes in Patients with Lung Cancer. Clin Cancer Res 2020;26:5701-8. [Crossref] [PubMed]
- Marzio A, Kurz E, Sahni JM, et al. EMSY inhibits homologous recombination repair and the interferon response, promoting lung cancer immune evasion. Cell 2022;185:169-183.e19. [Crossref] [PubMed]
- Deng J, Thennavan A, Dolgalev I, et al. ULK1 inhibition overcomes compromised antigen presentation and restores antitumor immunity in LKB1 mutant lung cancer. Nat Cancer 2021;2:503-14. [Crossref] [PubMed]
- Berland L, Heeke S, Humbert O, et al. Current views on tumor mutational burden in patients with non-small cell lung cancer treated by immune checkpoint inhibitors. J Thorac Dis 2019;11:S71-80. [Crossref] [PubMed]
- Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362:eaar3593. [Crossref] [PubMed]
- Heeke S, Benzaquen J, Long-Mira E, et al. In-house Implementation of Tumor Mutational Burden Testing to Predict Durable Clinical Benefit in Non-small Cell Lung Cancer and Melanoma Patients. Cancers (Basel) 2019;11:1271. [Crossref] [PubMed]
- Dagogo-Jack I, Schrock AB, Kem M, et al. Clinicopathologic Characteristics of BRG1-Deficient NSCLC. J Thorac Oncol 2020;15:766-76. [Crossref] [PubMed]
- Schoenfeld AJ, Bandlamudi C, Lavery JA, et al. The Genomic Landscape of SMARCA4 Alterations and Associations with Outcomes in Patients with Lung Cancer. Clin Cancer Res 2020;26:5701-8. [Crossref] [PubMed]
- Chiba Y, Kawanami T, Yamasaki K, et al. Hyper-progressive disease after immune checkpoint inhibitor in SMARCA4-deficient small-cell lung carcinoma. Respirol Case Rep 2020;8:e00667. [Crossref] [PubMed]
- Heeke S, Hofman P. Tumor mutational burden assessment as a predictive biomarker for immunotherapy in lung cancer patients: getting ready for prime-time or not? Transl Lung Cancer Res 2018;7:631-8. [Crossref] [PubMed]
- Gantzer J, Davidson G, Vokshi B, et al. Immune-Desert Tumor Microenvironment in Thoracic SMARCA4-Deficient Undifferentiated Tumors with Limited Efficacy of Immune Checkpoint Inhibitors. Oncologist 2022;27:501-11. [Crossref] [PubMed]
- Dong ZY, Zhong WZ, Zhang XC, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res 2017;23:3012-24. [Crossref] [PubMed]
- Papillon-Cavanagh S, Doshi P, Dobrin R, et al. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open 2020;5:e000706. [Crossref] [PubMed]
- Shang X, Li Z, Sun J, et al. Survival analysis for non-squamous NSCLC patients harbored STK11 or KEAP1 mutation receiving atezolizumab. Lung Cancer 2021;154:105-12. [Crossref] [PubMed]
- Marinelli D, Mazzotta M, Scalera S, et al. KEAP1-driven co-mutations in lung adenocarcinoma unresponsive to immunotherapy despite high tumor mutational burden. Ann Oncol 2020;31:1746-54. [Crossref] [PubMed]
- Xu X, Yang Y, Liu X, et al. NFE2L2/KEAP1 Mutations Correlate with Higher Tumor Mutational Burden Value/PD-L1 Expression and Potentiate Improved Clinical Outcome with Immunotherapy. Oncologist 2020;25:e955-63. [Crossref] [PubMed]
- Paz-Ares LG, Ramalingam SS, Ciuleanu TE, et al. First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J Thorac Oncol 2022;17:289-308. [Crossref] [PubMed]
- Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019;135:188-95. [Crossref] [PubMed]
- Saleh MM, Scheffler M, Merkelbach-Bruse S, et al. Comprehensive Analysis of TP53 and KEAP1 Mutations and Their Impact on Survival in Localized- and Advanced-Stage NSCLC. J Thorac Oncol 2022;17:76-88. [Crossref] [PubMed]


