
Identifying the risk factors and developing a predictive model for postoperative pelvic floor dysfunction in cervical cancer patients
Highlight box
Key findings
• Patients with cervical cancer have a high incidence of postoperative pelvic floor dysfunction. This study demonstrated that the risk factors for postoperative pelvic floor dysfunction in patients with cervical cancer included age greater than 65 years, open surgery, total hysterectomy, and radiotherapy.
What is known and what is new?
• Pelvic floor dysfunction is a common complication after cervical cancer surgery.
• We herein developed a predictive model to identify patients at high risk of pelvic floor dysfunction.
What is the implication, and what should change now?
• This model can help identify patients at high risk of developing pelvic floor dysfunction. Early management in such patients may be beneficial to reduce the incidence of pelvic floor dysfunction.
Introduction
The incidence of cervical cancer is approximately 4% and ranks sixth among all female malignancies in worldwide (1). While surgery is the main treatment for cervical cancer, postoperative pelvic floor dysfunction is a complication that can lead to severe impairment of a patient’s quality of life. A previous meta-study showed that postoperative urinary incontinence and constipation rates were as high as 34.1% and 11.1% after cervical cancer surgery (2). Pelvic floor dysfunction leads to the displacement of pelvic organs, which is linked to the dislocation and dysfunction of other pelvic organs. It is one of the top five chronic diseases affecting women’s quality of life, with clinical manifestations such as urinary incontinence and constipation (3,4). This current study explored the risk factors of postoperative pelvic floor dysfunction in cervical cancer patients. This information may be crucial in the prevention and treatment of patients with pelvic floor dysfunction. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-385/rc).
Methods
General patient information
A total of 282 cervical cancer patients who underwent surgery at the Wuhan No.7 Hospital from January 2020 to June 2022 was retrospectively enrolled. All patients were followed up after surgery. Patients were divided into a pelvic floor dysfunction group (n=92) and a control group (n=190) according to whether they developed pelvic floor dysfunction or not at 6 months post-surgery. The following inclusion criteria were applied: (I) patients were diagnosed with cervical cancer; (II) patients underwent surgical treatment in the Wuhan No.7 Hospital; and (III) patients were aged 18 years or greater. The following exclusion criteria were applied: (I) previous pelvic floor dysfunction; (II) lumbar spine diseases; (III) presentation of other malignant tumors; and (IV) loss to follow-up. The present study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Ethics Committee of the Wuhan No.7 Hospital (No. 202200874). Due to the retrospective nature of the study, patient informed consent was waived.
Treatment method
Following admission, patients completed the relevant examinations and received surgical treatment. Adjuvant therapy such as radiotherapy and chemotherapy were administered according to the pathological results.
Data collection
The following information was collated: age, body mass index (kg/m2), smoking history, history of alcoholism, hypertension, diabetes, hyperlipidemia, degree of tumor cell differentiation, pathological type, International Federation of Gynecology and Obstetrics (FIGO) stage, surgical methods, surgical resection range, radiation therapy, and chemotherapy.
Diagnostic criteria
Diagnostic criteria for pelvic floor dysfunction were defined as a disorder caused by pelvic floor support structures, injuries, and dysfunctions, including pelvic organ prolapse, urinary incontinence, fecal incontinence, sexual dysfunction, recurrent urinary tract, genital tract infections, and chronic pelvic pain.
Statistical analysis
SPSS26.0 (IBM, Chicago, USA) software was used for statistical data analysis and differences with a P value <0.05 were considered statistically significant. The measurement data of the two groups were expressed as mean ± standard deviation, and the differences between the two groups were analyzed by independent sample t-test. The count data of the two groups were expressed by n (%), and the chi-square test was used to analyze the differences between the two groups. Multivariate regression was used to analyze risk factors of pelvic floor dysfunction in patients with cervical cancer. The receiver operating characteristic (ROC) curve was used to analyze the predictive value of different indicators on pelvic floor dysfunction. The R4.0.3 statistical software was used to establish a prediction model for postoperative pelvic floor dysfunction in cervical cancer patients.
Results
Clinical features of the patients
The flow chart in Figure 1 shows the patient selection process. A total of 302 patients with cervical cancer were retrospectively analyzed. Two patients presented with previous pelvic floor dysfunction and were excluded. Another 2 patients were excluded due to presentation of other malignant tumors, 5 patients were excluded due to presentation of lumbar diseases, and 11 patients were lost to follow-up (Figure 1). Finally, 282 patients were included in the study, including 92 patients with pelvic floor dysfunction (Figure 1).
Analysis of the basic patient characteristics demonstrated that patients with and without pelvic floor dysfunction differed significantly in terms of age, surgical method, surgical resection range, and administration of radiotherapy (P<0.05; Table 1).
Table 1
| Variables | Pelvic floor dysfunction group (n=92) | Control group (n=190) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (years) | 60.93±11.77 | 54.37±11.54 | 4.451 | <0.001 |
| Age | 8.979 | 0.003 | ||
| >65 years | 36 (39.13) | 42 (22.11) | ||
| ≤65 years | 56 (60.87) | 148 (77.89) | ||
| Body mass index (kg/m2) | 23.06±2.89 | 23.53±2.74 | 1.303 | 0.194 |
| History of smoking | 6 (6.52) | 11 (5.79) | 0.059 | 0.809 |
| History of alcoholism | 4 (4.35) | 12 (6.32) | 0.449 | 0.503 |
| Hypertension | 8 (8.70) | 14 (7.37) | 0.152 | 0.697 |
| Diabetes | 5 (5.43) | 13 (6.84) | 0.205 | 0.650 |
| Hyperlipidemia | 12 (13.04) | 20 (10.53) | 0.390 | 0.532 |
| Tumor cell differentiation | 0.299 | 0.584 | ||
| Low | 21 (22.83) | 38 (20.00) | ||
| Medium to high | 71 (77.17) | 152 (80.00) | ||
| Type of pathology | 0.188 | 0.664 | ||
| Squamous | 70 (76.09) | 140 (73.68) | ||
| Adenocarcinoma | 22 (23.91) | 50 (26.32) | ||
| FIGO stage | 0.814 | 0.367 | ||
| Stage I | 32 (34.78) | 56 (29.47) | ||
| Stage II | 60 (65.22) | 134 (70.53) | ||
| Surgical methods | 9.273 | 0.002 | ||
| Open surgery | 21 (22.83) | 18 (9.47) | ||
| Minimally invasive surgery | 71 (77.17) | 172 (90.53) | ||
| Surgical resection range | 8.279 | 0.004 | ||
| Total hysterectomy | 57 (61.96) | 83 (43.68) | ||
| Partial hysterectomy | 35 (38.04) | 107 (56.32) | ||
| Radiotherapy | 6.100 | 0.014 | ||
| Yes | 44 (47.83) | 62 (32.63) | ||
| No | 48 (52.17) | 128 (67.37) | ||
| Chemotherapy | 0.213 | 0.644 | ||
| Yes | 54 (58.70) | 106 (55.79) | ||
| No | 38 (41.30) | 84 (44.21) |
Data are presented as mean ± standard deviation or n (%). FIGO, International Federation of Gynecology and Obstetrics.
Predictive value of age on postoperative pelvic floor dysfunction in cervical cancer patients
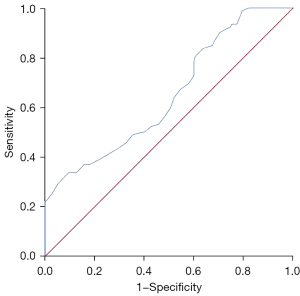
The ROC curve showed that age has a certain predictive value for postoperative pelvic floor dysfunction in cervical cancer patients, with an area under the curve of 0.650 (95% confidence interval: 0.582–0.719; P<0.001; Figure 2).
Risk factors for postoperative pelvic floor dysfunction in cervical cancer patients
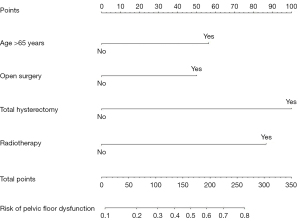
Age >65 years, open surgery, total hysterectomy, and radiotherapy were identified as risk factors of postoperative pelvic floor dysfunction in cervical cancer patients (P<0.05; Table 2).
Table 2
| Variables | B value | Standard error | Wald value | P value | Relative risk (95% CI) |
|---|---|---|---|---|---|
| Age >65 years | 0.617 | 0.292 | 4.456 | 0.035 | 1.854 (1.045–3.289) |
| Open surgery | 0.933 | 0.375 | 6.177 | 0.013 | 2.541 (1.218–5.302) |
| Total hysterectomy | 0.833 | 0.273 | 9.315 | 0.002 | 2.301 (1.347–3.929) |
| Radiotherapy | 0.595 | 0.275 | 4.694 | 0.030 | 1.813 (1.058–3.105) |
| Constant | −4.226 | 1.021 | 17.144 | <0.001 | 0.015 |
CI, confidence interval.
Establishment and validation of a postoperative pelvic floor dysfunction prediction model in cervical cancer patients
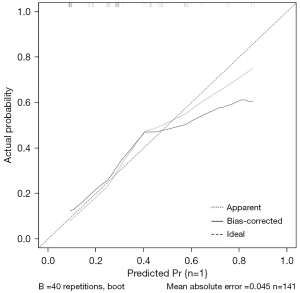
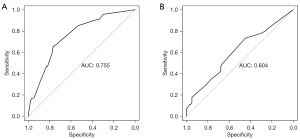
The R4.0.3 statistical software was used to randomly divide the dataset into a training dataset (n=141) and validation dataset (n=141). The area under the ROC curve was 0.755 (95% confidence interval: 0.673–0.837) for the training dataset, and 0.604 (95% confidence interval: 0.502–0.705) for the validation dataset. In the validation dataset, the model was tested with a Hosmer-Lemeshow Goodness-of-Fit test, with a chi-square value of 9.017 and a P value of 0.341 (Figures 3-6).
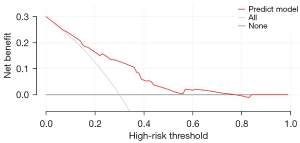
Discussion
Pelvic floor dysfunction is a common complication after cervical cancer surgery, with an incidence of up to 50% (2,5). The results herein demonstrated that age greater than 65 years, open surgery, total hysterectomy, and radiotherapy were risk factors of postoperative pelvic floor dysfunction in patients with cervical cancer (P<0.05). Furthermore, the prediction model established based on these relevant risk factors was valuable in identifying patients at high-risk of pelvic floor dysfunction after cervical cancer surgery.
Female pelvic floor dysfunction, including urinary incontinence and sexual dysfunction, is caused by pelvic floor support structure injuries and dysfunction, affecting approximately 50% of adult women (6). With the increase of the age, the pelvic floor support structure gradually deteriorates, and damage to the pelvic floor structure is further aggravated during cervical cancer surgery, resulting in pelvic floor dysfunction (7-10). A previous study demonstrated that increased age was a risk factor of pelvic floor dysfunction after cervical cancer surgery (3), which is consistent with the results of our present study. Pelvic surgery has a limited field of view, while the range of operation is deep and narrow. Furthermore, the nerves that affect pelvic function are often concentrated, and thus, surgical injuries that cause pelvic floor dysfunction are common. Open surgery for cervical cancer is more traumatic, and excessive stretching of the pelvic floor support structures for surgical visibility often results in pelvic floor dysfunction (11-13). Patients with total hysterectomy, who have a wide range of surgery, tend to have a late stage of FIGO, requiring extensive dissection of regional lymph nodes. Therefore, patients with total hysterectomy are more prone to structural and neurological damage, resulting in pelvic floor dysfunction (4,14). In recent years, with the development of minimally invasive technology, robotic surgery may be beneficial for reducing the incidence of pelvic floor dysfunction in patients with cervical cancer, but further research is still needed. Adhesions in the large intestine and pelvic wall may occur after radiotherapy for cervical cancer, further exacerbating the local inflammatory response after cervical cancer surgery, resulting in adhesions (15).
To better identify the patients at high-risk of postoperative pelvic floor dysfunction in cervical cancer patients, we established a nomogram prediction model for postoperative pelvic floor dysfunction. Nomograms are based on multivariate regression analysis and integrating multiple predictors, so as to express the interrelationship between various variables in a prediction model. Nomograms have been widely used in the diagnosis and prognosis of various diseases (16-21). In the present study, the area under the ROC curve of the training set of the predictive model was 0.755 (95% confidence interval: 0.673–0.837), indicating that the model has predictive valuable for postoperative pelvic floor dysfunction in cervical cancer patients. For patients who have developed pelvic floor dysfunction, both conservative treatment and surgery may be beneficial for improving their clinical symptoms (22).
Limitations
There were certain limitations to this study. First, this was a retrospectively clinical study and second, there were relatively few patients in the pelvic floor dysfunction group.
Conclusions
Prognosis and related indicators of different diseases are the focus of current research (23-26). The incidence of postoperative pelvic floor dysfunction in cervical cancer patients is high. Age greater than 65 years old, open surgery, total hysterectomy, and radiotherapy were identified as risk factors of postoperative pelvic floor dysfunction in cervical cancer patients. The prediction model developed herein is helpful for identifying patients at high-risk of pelvic floor dysfunction. Early management of such high-risk patients may be beneficial to reducing the incidence of pelvic floor dysfunction.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-385/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-385/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-385/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-385/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Ethics Committee of the Wuhan No.7 Hospital (No. 202200874). Due to the retrospective nature of the study, patient informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Shan X, Qian M, Wang L, et al. Prevalence of pelvic floor dysfunction and sexual dysfunction in cervical cancer survivors: a systematic review and meta-analysis. Int Urogynecol J 2023;34:655-64. [Crossref] [PubMed]
- Li M, Tian Q. Risk factors for postoperative pelvic floor dysfunction in patients with cervical cancer: evidences for management strategies. Transl Cancer Res 2021;10:4338-46. [Crossref] [PubMed]
- Liu DD, Xin J, Liu W, et al. Evaluation of Pelvic Floor Dysfunction by Pelvic Floor Ultrasonography after Total Hysterectomy for Cervical Cancer. Scanning 2022;2022:5914344. [Crossref] [PubMed]
- Miguel TP, Laurienzo CE, Faria EF, et al. Chemoradiation for cervical cancer treatment portends high risk of pelvic floor dysfunction. PLoS One 2020;15:e0234389. [Crossref] [PubMed]
- Monti M, Fischetti M, Santangelo G, et al. Urinary incontinence in women: state of the art and medical treatment. Minerva Obstet Gynecol 2021;73:135-9. [Crossref] [PubMed]
- Tosun G, Peker N, Tosun ÖÇ, et al. Pelvic floor muscle function and symptoms of dysfunctions in midwifes and nurses of reproductive age with and without pelvic floor dysfunction. Taiwan J Obstet Gynecol 2019;58:505-13. [Crossref] [PubMed]
- Tucker J, Grzeskowiak L, Murphy EM, et al. Do women of reproductive age presenting with pelvic floor dysfunction have undisclosed anal incontinence: A retrospective cohort study. Women Birth 2017;30:18-22. [Crossref] [PubMed]
- Leijonhufvud Å, Lundholm C, Cnattingius S, et al. Risk of surgically managed pelvic floor dysfunction in relation to age at first delivery. Am J Obstet Gynecol 2012;207:303.e1-7. [Crossref] [PubMed]
- Murad-Regadas SM, Rodrigues LV, Furtado DC, et al. The influence of age on posterior pelvic floor dysfunction in women with obstructed defecation syndrome. Tech Coloproctol 2012;16:227-32. [Crossref] [PubMed]
- Schiano di Visconte M, Azzena A. A 10-year retrospective cohort study to assess objective and subjective outcomes of combined stapled transanal rectal resection and urogynecological surgery for pelvic floor dysfunction. Arch Gynecol Obstet 2020;302:393-404. [Crossref] [PubMed]
- Ng SC, Chen CC, Cheng SH, et al. Escalating utilization of inpatient surgery for pelvic floor dysfunction in the elderly in Taiwan. Neurourol Urodyn 2019;38:1707-12. [Crossref] [PubMed]
- Montenegro M, Slongo H, Juliato CRT, et al. The Impact of Bariatric Surgery on Pelvic Floor Dysfunction: A Systematic Review. J Minim Invasive Gynecol 2019;26:816-25. [Crossref] [PubMed]
- Jin J, Wang M. Effects of Pelvic Floor Muscle Training Combined with Estriol on Pelvic Floor Dysfunction after Total Hysterectomy Applied in Perimenopause. Appl Bionics Biomech 2022;2022:6962542. [Crossref] [PubMed]
- Barcellini A, Dominoni M, Dal Mas F, et al. Sexual Health Dysfunction After Radiotherapy for Gynecological Cancer: Role of Physical Rehabilitation Including Pelvic Floor Muscle Training. Front Med (Lausanne) 2022;8:813352. [Crossref] [PubMed]
- Fu WY, Yuan H, Ye XQ, et al. Prediction of postpartum pelvic floor dysfunction with a nomogram model based on big data collected during pregnancy. Ann Palliat Med 2021;10:2143-51. [Crossref] [PubMed]
- Li P, Feng B, Liu Y, et al. Deep learning nomogram for predicting lymph node metastasis using computed tomography image in cervical cancer. Acta Radiol 2023;64:360-9. [Crossref] [PubMed]
- Zeng W, Huang L, Lin H, et al. Development and Validation of a Nomogram for Predicting Postoperative Distant Metastasis in Patients with Cervical Cancer. Med Sci Monit 2022;28:e933379. [Crossref] [PubMed]
- Yang S, Liu C, Li C, et al. Nomogram Predicting Lymph Node Metastasis in the Early-Stage Cervical Cancer. Front Med (Lausanne) 2022;9:866283. [Crossref] [PubMed]
- Xia X, Li D, Du W, et al. Radiomics Based on Nomogram Predict Pelvic Lymphnode Metastasis in Early-Stage Cervical Cancer. Diagnostics (Basel) 2022;12:2446. [Crossref] [PubMed]
- Wang M, Ma M, Yang L, et al. Development and validation of a nomogram for predicting pelvic lymph node metastasis and prognosis in patients with cervical cancer. Front Oncol 2022;12:952347. [Crossref] [PubMed]
- Schiavi MC, D’Oria O, Faiano P, et al. Vaginal Native Tissue Repair for Posterior Compartment Prolapse: Long-Term Analysis of Sexual Function and Quality of Life in 151 Patients. Female Pelvic Med Reconstr Surg 2018;24:419-23. [Crossref] [PubMed]
- Chen H, Meng X, Hao X, et al. Correlation Analysis of Pathological Features and Axillary Lymph Node Metastasis in Patients with Invasive Breast Cancer. J Immunol Res 2022;2022:7150304. [Crossref] [PubMed]
- Qiu Y, Chen H, Dai Y, et al. Nontherapeutic Risk Factors of Different Grouped Stage IIIC Breast Cancer Patients' Mortality: A Study of the US Surveillance, Epidemiology, and End Results Database. Breast J 2022;2022:6705052. [Crossref] [PubMed]
- Qiu Y, Chen Y, Zhu L, et al. Differences of Clinicopathological Features between Metaplastic Breast Carcinoma and Nonspecific Invasive Breast Carcinoma and Prognostic Profile of Metaplastic Breast Carcinoma. Breast J 2022;2022:2500594. [Crossref] [PubMed]
- Chen Y, Si H, Bao B, et al. Integrated analysis of intestinal microbiota and host gene expression in colorectal cancer patients. J Med Microbiol 2022; [Crossref] [PubMed]
(English Language Editor: J. Teoh)



