
Clinicopathological and prognosis significance of RIPK1 in patients with cervical squamous cell carcinoma: a retrospective cohort study
Highlight box
Key findings
• This study found that receptor interacting protein kinase 1 (RIPK1) was highly expressed in cervical cancer tissues. RIPK1 expression was significantly associated with several clinicopathologies. The multivariate analysis showed that RIPK1 was not an independent risk factor for PFS and OS in CSCC (P>0.05).
What is known and what is new?
• A large number of studies have confirmed that RIPK1 is related to the occurrence and development of tumors.
• This study identified high expression of RIPK1 in cervical squamous carcinoma and confirmed its clinicopathology.
What is the implication, and what should change now?
• This study showed that RIPK1 has a specific function in the occurrence and progression of cervical cancer and thus may be a new target for the treatment of cervical cancer. More research needs to be conducted to confirm the cellular level mechanism of RIPK1 activity in cervical cancer.
Introduction
Cervical cancer is one of the leading gynecologic cancers in women, and thus represents a huge challenge for health workers and researchers worldwide (1). The early screening and pervasive application of the human papillomavirus vaccine have led to a relative decline in the morbidity and mortality of cervical cancer patients. However, the 5-year overall survival (OS) rate of advanced cervical cancer patients remains low (2).
Standardized treatment is the main strategy used to delay the exacerbation of cervical cancer. In the past decade, precision medicine has highlighted the importance of individualized treatment based on gene targets. Cervical squamous cell carcinoma (CSCC) is a major pathologic subtype of cervical cancer (3). The identification of high-precision CSCC molecular markers and an understanding of the mechanisms involved in tumor progression is of great significance to the early diagnosis and precision treatment of CSCC.
Receptor interacting protein kinase 1 (RIPK1) is a regulatory protein of nuclear factor kappa B (NF-κB) signaling (4). It is one of the most important members of the receptor interacting protein (RIP) family. In recent years, RIPK1 kinase has emerged as a promising therapeutic target for the treatment of human neurodegenerative, autoimmune, and inflammatory disease (5). Currently, research suggests that RIPK1 polymorphisms are associated with the risk of cervical cancer in the Uygur population in China (6). Biologically, RIPK1 plays a significant role in the signal transduction of cell survival, apoptosis, and programmed necrosis through the activation of NF-κB, caspase-8, and reactive oxygen species production (7). Accumulative evidence has revealed the role of RIPK1 in diseases, such as breast cancer, ALS, and liver cancer (8-10). However, evidence on the expression of RIPK1 in CSCC and its relationship with clinicopathological features is limited; thus, in-depth research urgently needs to be conducted on the expression of RIPK1 in CSCC.
In the current study, clinicopathological information on the age, tumor size, vascular metastasis, lymph node metastasis, infiltrating depth, preoperative serum squamous cell carcinoma antigen (SCC-Ag) level, histological grade, and International Federation of Gynecology and Obstetrics (FIGO) stage of the patients with CSCC was collected. The expression of RIPK1 in CSCC was detected, and the association between RIPK1 expression and the aforementioned parameters were examined to identify the potential in the precision treatment of CSCC. We present this article in accordance with the REMARK reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-578/rc).
Methods
Patients
A retrospective cohort study was conducted, and PASS was used to calculate the sample size. A total of 100 CSCC tissue samples were collected from 100 patients who were diagnosed with CSCC and underwent curative surgery at The First Affiliated Hospital of Bengbu Medical College from January 1, 2019 to December 31, 2020. None of the participants received any preoperative chemoradiotherapy, and all of the participants were at the first visit to the hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Bengbu Medical College (No. 143,2021), and all the patients signed the informed consent form.
Specimen characteristics
The paraffin-embedded sections of the 100 clinical samples were treated with fixation, dehydration, transparency, wax transparency, embedding, sectioning, patching, staining, transparency, and paraffin embedding. The clinical pathological features of the patients enrolled in the study were obtained from the medical record system of The First Affiliated Hospital of Bengbu Medical College.
Study design
The patients completed telephone follow-up visits from the day of surgery until August 31, 2022. The follow-up period ranged from 20 to 43 months (average: 31 months). Progression-free survival (PFS) was defined as the time from diagnosis to recurrence, death, or last follow-up. OS was defined as the time from diagnosis to death or last follow-up. The surgical-pathological staging and histological grading were performed according to the 2018 FIGO staging standards (11,12). The clinicopathological characteristics included the age at diagnosis, tumor size, vascular metastasis, lymph node metastasis, invasion depth, SCC-Ag level, histological grade, and FIGO stage.
Assay methods
All the operations were conducted according to the manufacturers’ instructions. Anti-RIPK1 antibody (A7414, ABclonal Company, Wuhan, China), the DAB Chromogenic Substrate Kit Beijing Zhongshan Jinqiao Co., Ltd., Beijing, China) and the Strepavidin Peroxidase (SP) Detection Kit (Beijing Zhongshan Jinqiao Co., Ltd.) were used in the current study. The expression of RIPK1 in the CSCC tissues and normal tissue was detected by SP immunohistochemical staining. The steps were as follows: paraffin pathological tissue section, dewaxing and hydration, slice washing, blocking endogenous peroxidase antigen repair, adding primary antibody (dilution ratio 1:200) until the tissue was completely covered, adding secondary antibody (dilution ratio 1:1), DAB coloration, hematoxylin counterstaining, dehydration and drying, sealing the slices with neutral gum, and drying.
All the results were interpreted by a professional expert in pathology. RIPK1 staining was observed in the cytoplasm of the CSCC tissue, while the normal tissue was not stained. Five high magnifications (×200) were randomly observed for each CSCC tissue with 100 cells selected for counting. The positive proportion (PP) cell score was calculated according to the proportion of the cytoplasmic coloring and color development comprehensive score. PPs <25%, 25–50%, 50–75%, and 75–100% were defined as PP scores of 1, 2, 3, and 4 points, respectively. Cell staining of yellow, yellow-brown, and dark-brown were defined as cell staining intensity (SI) scores of 1, 2, and 3 points, respectively. The protein expression intensity was quantitated as the PP × SI.
Statistical analysis methods
SPSS20.0 statistical software was used for the data analysis. The Chi-square test and a 1-way analysis of variance were used to make comparisons between groups stratified based on RIPK1 expression. A Pearson linear correlation analysis was used to analyze the correlations between RIPK1 expression and the various clinicopathological indicators. A Cox survival regression analysis and Kaplan-Meier curves combined with a log-rank test were used to analyze the OS and PFS of patients. A multivariate logistic regression analysis was conducted to detect the risk factors for the impaired prognosis of CSCC. A P value <0.05 was considered statistically significant.
Results
Patient pathological characteristics
The patients had a mean age of 51.32±9.72 years (range, 25 to 72 years). Among the 100 patients, 41 patients were classified as FIGO stage I, and 59 patients were classified as FIGO stages II and III. As Table 1 shows, 79 patients had a histological grade G1/G2, and 21 had a histological grade G3. In addition, 19 (19%) of the patients had vascular metastasis, 30 (30%) had lymph node metastasis, and 75 (75%) had a ≥1/2 muscular layer infiltrating depth.
Table 1
| Variables | Cases | RIPK1 expression | χ2 | P | |
|---|---|---|---|---|---|
| Low | High | ||||
| FIGO stage | 6.533 | 0.011* | |||
| IA+IB | 41 | 16 | 25 | ||
| II+III | 59 | 14 | 45 | ||
| Histological grade | 3.676 | 0.159 | |||
| G1/G2 | 79 | 24 | 55 | ||
| G3 | 21 | 6 | 15 | ||
| Lymph node metastasis | 11.111 | 0.001** | |||
| No | 70 | 28 | 42 | ||
| Yes | 30 | 2 | 28 | ||
| Vascular metastasis | 0.894 | 0.344 | |||
| No | 81 | 26 | 55 | ||
| Yes | 19 | 4 | 15 | ||
| Infiltrating depth | 7.683 | 0.006** | |||
| <1/2 muscular layer | 25 | 13 | 12 | ||
| ≥1/2 muscular layer | 75 | 17 | 58 | ||
Analysis of the expression intensity of RIPK1 and the clinical and pathological indicators. *, was significantly correlated at the 0.05 level (bilateral); **, was significantly correlated at the 0.01 level (bilateral). RIPK1, receptor interacting protein kinase 1; FIGO, Federation of Gynecology and Obstetrics.
Expression of RIPK1 in the CSCC and normal samples
Figure 1 shows some representative images of RIPK1 immunostaining in the fresh CSCC tissue samples and normal cervical tissue samples. The immunostaining results showed that the expression of RIPK1 was negative in the normal cervical tissues and positive in the cytoplasm of the CSCC tissues. The immunostaining of the CSCC tissues was further categorized as yellow, yellow-brown, or dark-brown (Figure 1). The proportions of samples with low (yellow), moderate (yellow-brown), and high (dark-brown) immunostaining were 15%, 47%, and 38%, respectively. The differences were statistically significant (P<0.05).
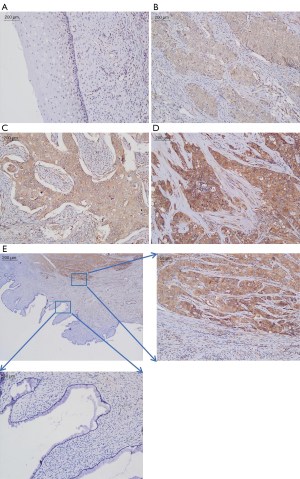
Association between RIPK1 expression and the clinicopathological features in patients with CSCC
As Table 2 shows, the PFS and OS differed significantly among the patients with different levels of invasion depth and FIGO stages (P<0.05). Vascular metastasis and the histopathologic grade did not differ significantly between the groups (P>0.05). Lymph node metastasis, the preoperative SCC-Ag level and age were associated with PFS (P<0.05) but were not associated with OS (P>0.05). The Kaplan-Meier analysis showed that the OS and PFS times of the high RIPK1 expression group were significantly lower than those of the low RIPK1 expression group (P<0.05, Figure 2). The Pearson linear regression showed that RIPK1 expression was positively correlated with the preoperative SCC-Ag level, tumor size, and age (all P<0.05, Table 3).
Table 2
| Variables | Cases | PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Value | 95% CI | χ2 | P | Value | 95% CI | χ2 | P | |||
| FIGO stage | 41.830 | 0.007** | 36.369 | 0.028* | ||||||
| IA+IB | 51 | 32±5 | 30–34 | 32±4 | 31–34 | |||||
| II+III | 49 | 30±7 | 29–32 | 31±7 | 29–32 | |||||
| Histological grade | 33.295 | 0.058 | 24.869 | 0.303 | ||||||
| G1/G2 | 79 | 31±6 | 29–32 | 31±6 | 30–33 | |||||
| G3 | 21 | 30±6 | 27–33 | 30±6 | 28–33 | |||||
| Lymph node metastasis | 40.375 | 0.001** | 31.293 | 0.090 | ||||||
| No | 70 | 31±6 | 30–33 | 31±6 | 30–33 | |||||
| Yes | 30 | 29±7 | 29–36 | 31±7 | 28–34 | |||||
| Vascular metastasis | 24.610 | 0.316 | 23.945 | 0.350 | ||||||
| No | 81 | 30±6 | 29–31 | 31±6 | 29–32 | |||||
| Yes | 19 | 36±7 | 29–36 | 33±7 | 30–37 | |||||
| Infiltrating depth | 48.966 | 0.001** | 43.930 | 0.004** | ||||||
| <1/2 muscular layer | 25 | 31±6 | 29–33 | 31±6 | 29–33 | |||||
| ≥1/2 muscular layer | 75 | 31±7 | 29–32 | 31±6 | 30–33 | |||||
| Age (years) | 34.421 | 0.044* | 26.588 | 0.227 | ||||||
| <60 | 81 | 31±6 | 29–32 | 31±6 | 30–33 | |||||
| ≥60 | 19 | 30±6 | 27–33 | 31±6 | 28–33 | |||||
| SCC-Ag level (μg/L) | 35.984 | 0.030* | 26.651 | 0.225 | ||||||
| <1.95 | 42 | 31±7 | 29–33 | 31±7 | 29–33 | |||||
| ≥1.95 | 58 | 30±6 | 29–32 | 31±6 | 29–33 | |||||
| RIPK1 expression | 48.903 | 0.001** | 52.091 | 0.000** | ||||||
| 1 | 6 | 26±6 | 20–32 | 26±6 | 20–32 | |||||
| 2 | 13 | 35±6 | 31–38 | 35±6 | 31–38 | |||||
| 4 | 11 | 30±5 | 27–34 | 30±5 | 27–34 | |||||
| 6 | 32 | 31±6 | 29–33 | 31±6 | 29–33 | |||||
| 8 | 5 | 25±3 | 21–28 | 25±3 | 21–28 | |||||
| 9 | 23 | 30±7 | 28–33 | 32±6 | 29–34 | |||||
| 12 | 10 | 31±7 | 26–36 | 31±7 | 27–36 | |||||
The relationship between RIPK1 expression intensity and clinical pathological indicators and patients’ PFS and OS; Value, standard deviation variance. *, was significantly correlated at the 0.05 level (bilateral); **, was significantly correlated at the 0.01 level (bilateral). SCC-Ag, squamous cell carcinoma antigen; RIPK1, receptor interacting protein kinase 1; PFS, progression-free survival; OS, overall survival; FIGO, Federation of Gynecology and Obstetrics.
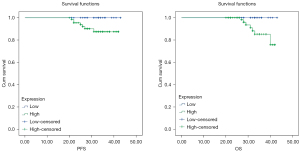
Table 3
| Variables | Pearson correlation | P |
|---|---|---|
| SCC-Ag level (μg/L) | 0.273 | 0.006** |
| Tumor size | 0.310 | 0.002** |
| Age (years) | 0.224 | 0.025* |
Person correlation analysis of the expression intensity of RIPK1 and the clinical indicators. *, was significantly correlated at the 0.05 level (bilateral); **, was significantly correlated at the 0.01 level (bilateral). RIPK1, receptor interacting protein kinase 1; SCC-Ag, squamous cell carcinoma antigen.
Multivariate regression analysis of the risk factors for decreased PFS and OS in patients with CSCC
The multivariate regression analysis showed that the preoperative SCC-Ag level, age, vascular metastasis, lymph node metastasis, the invasion depth, the FIGO stage, the histological grade, and RIPK1 expression were not independently associated with PFS and OS in patients with CSCC (P>0.05, Table 4).
Table 4
| Variables | PFS | OS | |||
|---|---|---|---|---|---|
| HR | P | HR | P | ||
| FIGO stage | 0.092 | 0.360 | 0.000 | 0.131 | |
| Histological grade | 0.080 | 0.088 | 0.000 | 0.210 | |
| Lymph node metastasis | 47,919.78 | 0.822 | 57.230 | 0.477 | |
| Vascular metastasis | 0.108 | 0.093 | 0.000 | 0.190 | |
| Infiltrating depth | 4.763 | 0.981 | 1,264.001 | 0.783 | |
| Age (years) | 0.777 | 0.804 | 0.067 | 0.192 | |
| SCC-Ag level (μg/L) | 1.071 | 0.9974 | 27.483 | 0.093 | |
| RIPK1 expression | 1.567 | 0.896 | 1.396 | 0.631 | |
Multi-factor Cox analysis. PFS, progression-free survival; OS, overall survival; CSCC, cervical squamous cell carcinoma; HR, hazard ratio; FIGO, Federation of Gynecology and Obstetrics; SCC-Ag, squamous cell carcinoma antigen; RIPK1, receptor interacting protein kinase 1.
Discussion
Study found that the expression of RIPK1 was significantly associated with the clinical stage, the histopathologic grade and lymph node metastasis but was not associated with age, tumor size and histopathologic type in breast cancer. The expression of RIPK1 was significantly associated with the invasion depth, clinical stage, histopathologic grade, and lymph node metastasis, but was not associated with sex, age and tumor size in esophageal squamous cell cancer. The expression of RIPK1 was significantly associated with the clinical stage, the histopathologic grade, and lymph node metastasis but was not associated with sex and age in colon cancer. In the present study, we assessed the expression of RIPK1 in CSCC tissues and analyzed the association between RIPK1 expression and the clinicopathological features of patients with CSCC. To the best of our knowledge, this was the first study to examine the expression of RIPK1 in CSCC.
Efforts, including surgery, radiotherapy, chemotherapy, and other treatments, have been made to reduce the mortality of cervical cancer patients; however, cervical cancer is still one of the most commonly diagnosed female cancers. In addition to the reduced survival time of patients, the quality of life of patients with cervical cancer significantly deteriorates. Notably, CSCC is one of the most common types of cervical cancer and has high mortality rates, and the age of onset in patients with CSCC is decreasing worldwide. CSCC are prone to develop tumor metastasis and have a high rate of recurrence. Thus, the present study sought to identify the specific biomarkers for CSCC and thus extend understandings of the pathogenesis of CSCC. Such findings will be of great clinical value.
RIPK1 is a class of serine/threonine protein kinases and is well recognized as a multidomain protein with an N-terminal kinase domain, an intermediate domain, and a C-terminal death domain (13). The RIP family plays an essential role in the inflammation and activation of the pathways involved in cell death (14). RIPK1 contains a death domain through which it can interact with FADD to recruit caspase 8 and FLIP (and also caspase 10 in humans). Previous study has shown that complexes that contain necrosomes, such as RIPK1 and/or RIPK3, can activate caspase 8 and induce apoptosis (15). However, RIPK1 kinase activity-related cell apoptosis is mediated by the NF-κB essential modulator, which is the regulatory subunit of the inhibitor of the NF-κB kinase (IKK) complex. IKKα and IKKβ have been reported to be other complexes involved (16). We speculated that not only NF-κB-dependent but also NF-κB-independent mechanisms, such as IKKα- or IKKβ-mediated RIPK1 phosphorylation and NF-κB essential modulator-mediated disruption of necrosome formation. However, the physiological circumstances in which RIPK1-dependent apoptosis becomes predominant are poorly understood. The inhibition of RIPK1 kinase activity using improved necrostatin-1 (R-7-Cl-O-Nec-1, Nec-1s) and trials with animal models of RIPK1 kinase-dead mutant mice have shown the efficacy of RIPK1-inhibitors in the treatment of diseases (17-19). However, the mechanism of RIPK1 in CSCC requires further investigation.
In the current study, the immunostaining results showed that the expression of RIPK1 was positive in the CSCC tissues but negative in the normal tissues. Further, the Pearson linear regression analysis revealed a positive correlation between RIPK1 expression and certain clinicopathological features of patients. Additionally, the relationship between RIPK1 expression and PFS and OS was revealed. The Kaplan-Meier survival curve analysis showed that the CSCC patients with high RIPK1 expression levels had worse OS and PFS. The multivariate regression analysis showed that RIPK1 was not an independent prognostic factor for CSCC. These results provide evidence that RIPK1 plays a significant role in the progression of CSCC and might serve as a treatment target for CSCC.
This study had a number of limitations. First, the sample size was small, and the data was collected from a single center. Second, the follow-up time was relatively short. Third, only PFS and OS were considered in examining patient prognosis, and no information on mortality was collected. In the future, clinical research with larger sample sizes and prolonged follow-up periods needs to be conducted to extend understandings of RIPK1 and its association with the progression of CSCC.
Conclusions
In conclusion, our findings demonstrated that an increased expression of RIPK1 in CSCC is closely associated with patients’ clinicopathological features and a worse prognosis. The expression of RIPK1 might be involved in the progression of CSCC and might be used to predict the prognosis of CSCC patients. RIPK1 may be a target for the treatment of CSCC.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-578/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-578/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-578/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-578/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Bengbu Medical College (No. 143,2021), and all the patients signed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 2001;358:781-6. [Crossref] [PubMed]
- Bhatla N, Aoki D, Sharma DN, et al. Cancer of the cervix uteri. Int J Gynaecol Obstet 2018;143:22-36. [Crossref] [PubMed]
- Liu Z, Chan FK. Regulatory mechanisms of RIPK1 in cell death and inflammation. Semin Cell Dev Biol 2021;109:70-5. [Crossref] [PubMed]
- Degterev A, Ofengeim D, Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci U S A 2019;116:9714-22. [Crossref] [PubMed]
- Tuoheti Z, Han L, Husaiyin S, et al. RIPK1 polymorphisms alter the susceptibility to cervical Cancer among the Uyghur population in China. BMC Cancer 2020;20:299. [Crossref] [PubMed]
- Liu L, Lalaoui N. 25 years of research put RIPK1 in the clinic. Semin Cell Dev Biol 2021;109:86-95. [Crossref] [PubMed]
- Baik JY, Liu Z, Jiao D, et al. ZBP1 not RIPK1 mediates tumor necroptosis in breast cancer. Nat Commun 2021;12:2666. [Crossref] [PubMed]
- Ito Y, Ofengeim D, Najafov A, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 2016;353:603-8. [Crossref] [PubMed]
- Schneider AT, Gautheron J, Feoktistova M, et al. RIPK1 Suppresses a TRAF2-Dependent Pathway to Liver Cancer. Cancer Cell 2017;31:94-109. [Crossref] [PubMed]
- Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 2019;145:129-35. [Crossref] [PubMed]
- Corrigendum to. Revised FIGO staging for carcinoma of the cervix uteri Int J Gynaecol Obstet 2019;147:279-80. [Int J Gynecol Obstet 145(2019) 129-135]. [Crossref] [PubMed]
- Wang Q, Fan D, Xia Y, et al. The latest information on the RIPK1 post-translational modifications and functions. Biomed Pharmacother 2021;142:112082. [Crossref] [PubMed]
- Kondylis V, Kumari S, Vlantis K, et al. The interplay of IKK, NF-κB and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation. Immunol Rev 2017;277:113-27. [Crossref] [PubMed]
- Vlantis K, Wullaert A, Polykratis A, et al. NEMO Prevents RIP Kinase 1-Mediated Epithelial Cell Death and Chronic Intestinal Inflammation by NF-κB-Dependent and -Independent Functions. Immunity 2016;44:553-67. [Crossref] [PubMed]
- Dondelinger Y, Jouan-Lanhouet S, Divert T, et al. NF-κB-Independent Role of IKKα/IKKβ in Preventing RIPK1 Kinase-Dependent Apoptotic and Necroptotic Cell Death during TNF Signaling. Mol Cell 2015;60:63-76. [Crossref] [PubMed]
- Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol 2014;35:14-23. [Crossref] [PubMed]
- Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005;1:112-9. [Crossref] [PubMed]
- Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 2008;4:313-21. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


