
Unearthing a novel tumor suppressor function of ATOH8 in hepatocellular carcinoma: role in acquisition of cancer stem cell-like features
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer worldwide and the third most common cause of cancer-related deaths (1). Surgical resection and liver transplantation are available for early-stage HCC, yet only a small proportion of patients are amenable to surgery as most patients are diagnosed at advanced stages. Although chemotherapy is often administered to patients with inoperable HCC as an adjuvant therapy, relapse rates and mortality rates remain high due to the strong tendency for tumors to develop drug resistance. Identifying novel prognostic markers and therapeutic targets for HCC is therefore essential for managing HCC and improving the long-term survival of patients. In a recent study published in Gastroenterology (2), atonal homolog 8 (ATOH8), which plays a crucial role in development, has been demonstrated to function as a tumor suppressor in HCC and might serve as a novel therapeutic target.
Development is finely orchestrated by spatio-temporal regulation of the expressions of a broad repertoire of genes. The basic helix-loop-helix (bHLH) proteins form a large superfamily of transcription regulators that are highly conserved from yeast to human and function in critical developmental processes. The atonal homolog (ATOH) proteins, characterized by a bHLH domain that typically binds to a consensus sequence (CANNTG) E-box (3), are involved in a wide range of biological processes including development of inner ear hair cells (4), retina ontogenesis (5) and skeletal muscle development (6).
ATOH8/MATH6, a novel member of the bHLH gene family, contains 321 amino acids with one bHLH domain and shares structural similarity with the Drosophila proneural gene ATONAL (7). ATOH8 has been implicated in the development of skeletal muscle (6), liver (8) and pancreas (9). Song et al. recently reported down-regulation of ATOH8 in HCC tissues and correlation with poor patients’ survival (2). Using a series of in vitro and in vivo functional experiments, ectopic expression of ATOH8 was found to significantly inhibit the proliferative, migratory and invasive capabilities of HCC cells. Intriguingly, increased CD133+ cell population in HCC cell lines resulting from ATOH8 depletion was revealed to possess cancer stem cell (CSC) properties including enhanced abilities of self-renewal, differentiation and acquisition of chemoresistance. Mechanistically, ATOH8 transcriptionally repressed stem cell-associated genes NANOG, OCT4 and CD133 (Figure 1). Song et al. (2) further showed introduction of ATOH8 increased the chemosensitivity of tumor cells to 5-fluorouracil and cisplatin treatment, suggesting ATOH8 might serve as a potential therapeutic target in HCC.
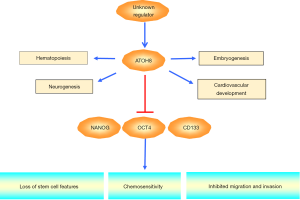
Given the importance of ATOH proteins in the regulation of various developmental processes, it comes with no surprise that deregulation of their modes of transcriptional regulation and action will lead to pathological conditions. For instance, deletion of the remote enhancer of ATOH7 has been documented to be associated with nonsyndromic congenital retinal nonattachment (10). Another key member of the bHLH gene family, ATOH1, is implicated in the progression of tumor. Although like ATOH8, ATOH1 has been suggested to function as a tumor suppressor by modulating proliferation and apoptosis in colon cancer (11), a recent study demonstrated contrasting data showing that ATOH1 stabilized by tumor necrosis factor α (TNFα) could induce a cancer stem cell phenotype leading to the acquisition of chemoresistance (12). CSCs are believed to exist in a wide array of tumors responsible for tumor heterogeneity and poor patients’ survival (13). In addition to providing the first piece of evidence reporting the tumor suppressor role of ATOH8, Song et al. (2) suggested ATOH8 knockdown is capable of stimulating HCC cells with non-CSCs to CSCs reprogramming primarily through the relief of transcriptional suppression of NANOG and OCT4, thereby providing further understanding to the roles of CSCs in tumor progression and supportive evidence to a recent study demonstrating a pivotal role of ATOH8 in human embryonic endothelial differentiation and function (14). This is also in line with accumulating findings documenting NANOG and OCT4 are broadly expressed in various cancer types and could induce cancer cells to dedifferentiate into CSC-like cells (15,16). Other members of the ATOH family have also been implicated in cell differentiation. ATOH1 has been shown to be indispensable for tuft cell differentiation (17). Using genetically modified mouse lines with differing extents of retinal ganglion cell loss, ATOH7 was documented to regulate the differentiation of retina into neurons (18). The role of ATOH8 as a transcriptional repressor was also supported by an investigation showing ATOH8 lacks a transactivation domain and possesses intrinsic repressor activity (3). Uncovering the tumor suppressing role of ATOH8 opens the avenue for establishing ATOH8 as a novel therapeutic target in HCC.
In order to determine whether overexpression of ATOH8 is capable of re-sensitizing HCC cells to chemotherapy, the authors adopted a lentiviral-based approach and suggested ATOH8 restoration has great therapeutic potential in HCC treatment (2). Although gene therapy using lentiviruses offers numerous advantages over conventional retroviral gene delivery systems including stable and prolonged gene expression, the clinical use of lentiviral-based vectors has raised concerns regarding safety and ethical issues awaiting further in-depth investigations (19). In fact, deciphering the mechanism underlying the down-regulation of ATOH8, which has not been addressed in the current study, might provide an alternate way out. Genetic alterations (e.g., mutations) and epigenetic modulations (e.g., hypermethylation of promoter region) have been associated with the silencing of a wide array of tumor suppressor genes. Uncovering the cause of loss of ATOH not only enables a more comprehensive understanding of the functional role of ATOH8, but also sheds light on the development of alternate therapeutic strategies. For instance, if DNA methylation was accountable for the loss of ATOH8, the drug decitamine (5-aza-2’-dexoycytidine), which has been reported to reverse the aberrant hypermethylation of silenced genes and deliver favorable clinical responses in patients with acute myelogenous leukemia (20), might serve as a treatment approach for HCC by restoring the expression of ATOH8 and re-sensitizing cancer cells to chemotherapy.
In summary, current findings demonstrated a novel tumor suppressor role of ATOH8 in the progression of HCC. ATOH8 silencing enables HCC cells to reprogram from non-CSCs to CSCs. Furthermore, introduction of ATOH8 enhances the sensitivity of HCC cells to 5-fluorouracil and cisplatin thereby implicating a potential therapeutic value of ATOH8.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Fei Pan (Department of Gastroenterology and Hepatology, Division of Internal Medicine, PLA Medical School & PLA General Hospital, Beijing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.05.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49. [Crossref] [PubMed]
- Song Y, Pan G, Chen L, et al. Loss of ATOH8 Increases Stem Cell Features of Hepatocellular Carcinoma Cells. Gastroenterology 2015;149:1068-81.e5. [Crossref] [PubMed]
- Ejarque M, Altirriba J, Gomis R, et al. Characterization of the transcriptional activity of the basic helix-loop-helix (bHLH) transcription factor Atoh8. Biochim Biophys Acta 2013;1829:1175-83.
- Stojanova ZP, Kwan T, Segil N. Epigenetic regulation of Atoh1 guides hair cell development in the mammalian cochlea. Development 2015;142:3529-36. [Crossref] [PubMed]
- Skowronska-Krawczyk D, Chiodini F, Ebeling M, et al. Conserved regulatory sequences in Atoh7 mediate non-conserved regulatory responses in retina ontogenesis. Development 2009;136:3767-77. [Crossref] [PubMed]
- Balakrishnan-Renuka A, Morosan-Puopolo G, Yusuf F, et al. ATOH8, a regulator of skeletal myogenesis in the hypaxial myotome of the trunk. Histochem Cell Biol 2014;141:289-300. [Crossref] [PubMed]
- Jarman AP, Grau Y, Jan LY, et al. Atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell 1993;73:1307-21. [Crossref] [PubMed]
- Kautz L, Meynard D, Monnier A, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood 2008;112:1503-9. [Crossref] [PubMed]
- Lynn FC, Sanchez L, Gomis R, et al. Identification of the bHLH factor Math6 as a novel component of the embryonic pancreas transcriptional network. PLoS One 2008;3:e2430 [Crossref] [PubMed]
- Ghiasvand NM, Rudolph DD, Mashayekhi M, et al. Deletion of a remote enhancer near ATOH7 disrupts retinal neurogenesis, causing NCRNA disease. Nat Neurosci 2011;14:578-86. [Crossref] [PubMed]
- Bossuyt W, Kazanjian A, De Geest N, et al. Atonal homolog 1 is a tumor suppressor gene. PLoS Biol 2009;7:e39 [Crossref] [PubMed]
- Fukushima K, Tsuchiya K, Kano Y, et al. Atonal homolog 1 protein stabilized by tumor necrosis factor α induces high malignant potential in colon cancer cell line. Cancer Sci 2015;106:1000-7. [Crossref] [PubMed]
- Lobo NA, Shimono Y, Qian D, et al. The biology of cancer stem cells. Annu Rev Cell Dev Biol 2007;23:675-99. [Crossref] [PubMed]
- Fang F, Wasserman SM, Torres-Vazquez J, et al. The role of Hath6, a newly identified shear-stress-responsive transcription factor, in endothelial cell differentiation and function. J Cell Sci 2014;127:1428-40. [Crossref] [PubMed]
- Jeter CR, Yang T, Wang J, et al. Concise Review: NANOG in Cancer Stem Cells and Tumor Development: An Update and Outstanding Questions. Stem Cells 2015;33:2381-90. [Crossref] [PubMed]
- Pan GJ, Chang ZY, Schöler HR, et al. Stem cell pluripotency and transcription factor Oct4. Cell Res 2002;12:321-9. [Crossref] [PubMed]
- Gerbe F, van Es JH, Makrini L, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 2011;192:767-80. [Crossref] [PubMed]
- Kiyama T, Li H, Gupta M, et al. Distinct neurogenic potential in the retinal margin and the pars plana of mammalian eye. J Neurosci 2012;32:12797-807. [Crossref] [PubMed]
- Connolly JB. Lentiviruses in gene therapy clinical research. Gene Ther 2002;9:1730-4. [Crossref] [PubMed]
- Issa JP, Garcia-Manero G, Giles FJ, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies. Blood 2004;103:1635-40. [Crossref] [PubMed]


