
MET exon 14 mutation: another actionable genomic variation in patients with advanced NSCLC
In their recent work published in the Journal of Clinical Oncology, Awad and colleagues presented the interesting results of next-generation sequencing analysis conducted on tissue from 6,376 cancer patients, with a special focus on the description of MET exon 14 mutations in patients with non-squamous non-small cell lung cancer (NSCLC) (1). Nowadays, using the molecular platforms that have been developed, detailed information about the presence or absence of a very high number of different molecular alterations can be acquired simultaneously and in a very short time. Thanks to the availability of targeted drugs, much of this information is not only useful to increase our knowledge about molecular characteristics of different tumors, but it can be also useful to select patients as candidates for the treatment with specific agents.
Awad and colleagues emphasize that patients with MET mutation are, on average, older than those with Epidermal Growth Factor Receptor (EGFR) mutation or KRAS mutation: namely, median age was 72.5 years, compared to 61 years for patients with EGFR mutation and 65 years for patients with KRAS mutation. Furthermore, more than half of the 28 MET exon 14 mutations were found in patients who had a positive history of smoking, with more than 10 pack-years. In these years, we have learned that the selection of patients for molecular analyses established as part of routine clinical practice, such as EGFR and ALK, cannot be based solely on clinical characteristics (2,3). EGFR mutations are more common in women, in younger subjects, in never smokers and in subjects from East Asia, but current guidelines do recommend testing for EGFR mutation in all patients with non-squamous advanced NSCLC, and even in patients with squamous tumor if never or former light smokers (2). From this point of view, the description of clinical characteristics commonly associated with a specific alteration will probably not definitely restrict the selection of patients to test. However, showing that a targetable molecular alteration is relatively common in older subjects and in patients with a positive history of smoking, has a conceptual great relevance. The number of “slices” corresponding to defined molecular alterations in the pie chart of patients with advanced NSCLC is constantly increasing, and this corresponds to an increase in therapeutic opportunities.
From a regulatory point of view, the description of “druggable” alterations in a limited subgroup of patients has relevant implications for the authorization of new drugs in clinical practice. MET exon 14 mutation is described by Awad in about 3% of non-squamous lung cancer patients. At first sight, this could be judged a “rare” occurrence, but it should be considered a relatively “common” alteration, if compared to many others that are represented in less than 1% of patients. In March 2016, US Food and Drug administration has approved crizotinib for the treatment of ROS-1 positive advanced NSCLC. This approval follows the demonstration of activity (in terms of overall response rate) within a multi-center, single-arm study of 50 patients selected for the presence of the specific alteration (4). Given the rarity of the alteration (ROS-1 alteration is present in about 1% of advanced NSCLC), the conduction of a randomized trial is highly unlikely. Probably, the same regulatory process will happen for other molecular alterations.
How to deal with the molecular information obtained in the analysis by Awad and colleagues, given that there is no drug specifically approved for the use in clinical practice for that indication? Does it make sense to acquire information about a wide panel of molecular alterations, when the potentially active drugs cannot be officially prescribed in that patient? Ideally, when a next-generation sequencing testing is proposed to a patient and an “actionable” alteration is diagnosed, the patient should have the opportunity to receive the corresponding drug. In the report by Awad, one patient with MET exon 14 mutation, in association with high-level MET amplification, after disease progression during first-line chemotherapy, received crizotinib obtaining a clinically relevant partial response. Several other case reports have been recently published, describing the activity of crizotinib or other drugs (capmatinib, cabozantinib) in patients with MET exon 14 mutation positive advanced NSCLC (5-12). All the cases published to date are reported in Table 1. Of note, most of the patients obtained an objective response with the targeted treatment.
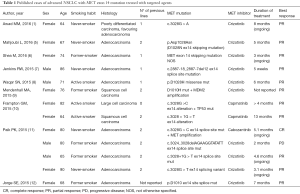
Full table
Interestingly, the National Comprehensive Cancer Network NSCLC Guidelines Panel, moving from the principle that broad molecular profiling can be a key component of the improvement of care of patients with NSCLC, strongly endorses broader molecular profiling with the goal of identifying rare driver mutations for which effective drugs may already be available, or to appropriately counsel patients regarding the availability of clinical trials (13). Of note, at the moment, patients who are diagnosed with MET exon 14 mutation could not receive crizotinib in clinical practice, according to official authorization for drug use, and participation in a clinical trial remain the only opportunity to receive a targeted treatment. In Italy, a prospective study testing the activity of crizotinib in patients with advanced NSCLC, selected for the presence of ROS-1 translocation, MET amplification or MET exon 14 mutation is currently ongoing (METROS, ClinicalTrials.gov Identifier NCT02499614). This kind of trials carry two important values: one immediate value for patients who are eligible and can enter the trial, because they will be able to receive the drug, that would be otherwise not available. The second important value is to produce scientific evidence about the activity of the experimental drug in patients selected for these rare alterations. We recognize that even a single case report, like those summarized in Table 1, can be important to give the “proof of principle” of drug activity in that setting, but there is no doubt that a well conducted trial, with adequate sample size corresponding to a predefined hypothesis of activity, will produce more robust evidence for the scientific community and for the treatment of future patients. We strongly believe that the same effort, implying an active role of both academic Institutions and pharmaceutical companies, should be made for all the rare molecular alterations that can be now diagnosed thanks to the advances in laboratory diagnostics. From this point of view, it is encouraging that in 2015, both American Society of Clinical Oncology (ASCO) and US National Cancer Institute (NCI) announced interesting plans for precision medicine trials, with the double aim that we described above: (I) offering an instrument for giving potential benefit to patients based on the results of wide genomic testing; and (II) increasing scientific knowledge about drug activity in selected subgroups of patients. The ASCO trial TAPUR (Targeted Agent and Profiling Utilization Registry, ClinicalTrials.gov Identifier NCT02693535) is designed to collect information on activity of commercially available targeted agents in patients diagnosed with a range of cancer types (advanced solid tumors, non-Hodgkin lymphoma, multiple myeloma), which are positive for the presence of a genomic alteration known to be a drug target. As explained by ASCO Chief Medical Officer Richard L. Schilsky, this trial will give the opportunity to acquire information about molecular targets and potential treatments that have some report of activity but are not formally available in clinical practice (14). Without this kind of clinical trials, this source of scientific knowledge is probably lost, because there is no structured mechanism (unless the publication of sporadic case reports) to learn from the treatment of patients who have the chance of receiving targeted drugs in off-label settings. As of March 2016, the TAPUR study is evaluating 12 different drugs and drug combinations, produced by several different pharmaceutical companies: in one of these groups, patients selected for the presence of MET mutations are treated, along with ALK positive and ROS-1 positive cases, with crizotinib. Primary outcome measure is the objective response rate. Another interesting project testing the activity of rationally selected biomarker/targeted therapy combinations is the MATRIX trial (National Lung Matrix Trial: Multi-drug, Genetic Marker-directed, Non-comparative, Multi-centre, Multi-arm Phase II Trial in Non-small Cell Lung Cancer, ClinicalTrials.gov Identifier NCT02664935), sponsored by the University of Birmingham, UK (15). Within the Cancer Research UK Stratified Medicine Programme 2, a large volume national molecular pre-screening is ongoing, with the aim of selecting patients with advanced NSCLC who are eligible to be included in the MATRIX trial. At study initiation, there were eight drugs being used to target 18 molecular cohorts: among patients in the molecular cohort who receive crizotinib, MET exon 14 mutation has been added to MET amplification and ROS1 rearrangement. Similarly, the NCI-MATCH (Molecular Analysis for Therapy Choice, ClinicalTrials.gov Identifier NCT02465060) trial, coordinated by the ECOG-ACRIN Cancer Research Group, is planned to incorporate more than 20 different drugs or drug combinations, each directed against a specific gene mutation, with the aim of treating the highest proportion of patients with a targeted therapy. MET exon 14 mutation is not listed among the actionable mutations included in the trial as of January 2016 (http://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-match#7), but additional arms are expected to open during the conduction of the study. In fact, one advantage of the “flexible” design of this kind of trials is the opportunity of adding new molecular alterations among those eligible for inclusion, corresponding to further cohorts of patients who receive a targeted treatment.
In conclusion, the paper by Awad and colleagues describes a novel molecular subtype of advanced NSCLC. This has relevant implications for the conduction of prospective clinical trials, with the aim of validating the role of MET exon 14 mutations as a therapeutic target. Consequently, in the near future, there could be a well-defined role for molecular testing of these mutations in clinical practice.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Qing-Yuan Huang (Department of Thoracic Surgery, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.04.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J Clin Oncol 2016;34:721-30. [Crossref] [PubMed]
- Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii27-39. [Crossref] [PubMed]
- Kerr KM, Bubendorf L, Edelman MJ, et al. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol 2014;25:1681-90. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Mahjoubi L, Gazzah A, Besse B, et al. A never-smoker lung adenocarcinoma patient with a MET exon 14 mutation (D1028N) and a rapid partial response after crizotinib. Invest New Drugs 2016;34:397-8. [Crossref] [PubMed]
- Shea M, Huberman MS, Costa DB. Lazarus-Type Response to Crizotinib in a Patient with Poor Performance Status and Advanced MET Exon 14 Skipping Mutation-Positive Lung Adenocarcinoma. J Thorac Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Jenkins RW, Oxnard GR, Elkin S, et al. Response to Crizotinib in a Patient With Lung Adenocarcinoma Harboring a MET Splice Site Mutation. Clin Lung Cancer 2015;16:e101-4. [Crossref] [PubMed]
- Waqar SN, Morgensztern D, Sehn J. MET Mutation Associated with Responsiveness to Crizotinib. J Thorac Oncol 2015;10:e29-31. [Crossref] [PubMed]
- Mendenhall MA, Goldman JW. MET-Mutated NSCLC with Major Response to Crizotinib. J Thorac Oncol 2015;10:e33-4. [Crossref] [PubMed]
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850-9. [Crossref] [PubMed]
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842-9. [Crossref] [PubMed]
- Jorge SE, Schulman S, Freed JA, et al. Responses to the multitargeted MET/ALK/ROS1 inhibitor crizotinib and co-occurring mutations in lung adenocarcinomas with MET amplification or MET exon 14 skipping mutation. Lung Cancer 2015;90:369-74. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer (Version 4.2016) Available online: www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Tsimberidou AM, Eggermont AM, Schilsky RL. Precision cancer medicine: the future is now, only better. Am Soc Clin Oncol Educ Book 2014;61-9. [Crossref] [PubMed]
- Middleton G, Crack LR, Popat S, et al. The National Lung Matrix Trial: translating the biology of stratification in advanced non-small-cell lung cancer. Ann Oncol 2015;26:2464-9. [PubMed]


