
Disease prognosis and therapeutic strategies in patients with advanced non-small cell lung cancer (NSCLC): a 6-year epidemiological study between 2015–2021
Highlight box
Key findings
• Risk of mortality in non-small cell lung cancer (NSCLC) patients was associated with being more than 64 years old male smoker.
• Two-thirds of NSCLC patients died at a median follow-up of 18 months from the date of diagnosis, with a median survival time of 123 days.
What is known and what is new?
• Sadly, over half of NSCLC patients die within 1 year of lung cancer diagnosis because of its late discovery.
• In this 6-year cross-sectional study, we found that simultaneous use of chemotherapy and immunotherapy had a significant treatment response.
• Disease progression was higher with pleural and brain metastasis.
What is the implication, and what should change now?
• Continued research into new drugs and combination therapies is required to expand the clinical benefit to a broader patient population and to improve outcomes in NSCLC.
Introduction
Lung cancer is one of the most fatal types of cancers worldwide. In men, it is the main leading cause of death, while for women it is the second leading cause of cancer death after breast cancer. An increasing pattern of cancer occurrence has been shown since 2012; this is due to the growth and aging of the population, as well as an increase in the prevalence of established risk factors such as smoking, occupational exposure, physical inactivity and being overweight (1). In Saudi Arabia, only 14% of cases are diagnosed at an early stage, and despite pharmacological advancements, over half of the patients with lung cancer die within 1 year of diagnosis. Ninety percent of lung cancer cases are diagnosed with metastatic disease, where choices of treatment and prognosis are limited, with a 5-year survival rate between 12–15% (2).
There are two major subtypes of lung cancer: small cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC) which is more prevalent and accounts for 85% of all lung cancer cases (3). NSCLC is further defined by molecular and histological profiling into three distinct types: squamous-cell carcinoma, adenocarcinoma, and large-cell carcinoma (4). The most common type is adenocarcinoma lung cancer which comprises 40% of all lung cancer cases for both genders. It arises from small airway epithelial type II alveolar cells, which secrete mucus and other substances (5). Adenocarcinoma localizes in the lung periphery, while large-cell carcinoma tends to begin in the central part of the lungs (6). In addition to histological differences between each subtype, advanced NSCLC is also defined by a variety of cell biomarkers, including EGFR, ALK, ROS1, MET, HER2 and BRAF, as well as PD-L1. Hence, the treatment of advanced NSCLC becomes more complex with different treatment goals, including standard gold-standard chemotherapy, targeted molecular inhibitors and immunotherapy. Patients with a confirmed diagnosis of NSCLC require a principal set of imaging studies as well as blood counts and chemistries, including serum calcium, kidney function and liver function testing to determine the definitive stage and possible management options (7).
Cigarette smoking is a primary relative risk factor for lung cancer due to the presence of considerable carcinogens in tobacco smoke. Smokers have as much as a 30-fold increased risk of developing cancer compared to non-smokers. Thus, there is an emphasis on the implementation of new smoking cessation programs, screening methods and improved lung cancer diagnostics approaches, until lung cancer is both a preventable and a curable disease (8).
Clinical features and findings of NSCLC cases discovered at an advanced stage have not yet reviewed or identified for the Saudi population. Challenges in disease management arise because of late discovery. In an attempt to review and identify the challenges associated with the screening, diagnosis, management, and outcome of Saudi patients with advanced NSCLC and to be better prepared to control this fatal disease, this study aimed to assess the prevalence, clinicopathological characteristics and clinical outcomes of advanced NSCLC patients in Saudi Arabia. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1816/rc).
Methods
Study cohort
This is a cohort study conducted at King Khalid University Hospital (KKUH) and King Fahd Medical City (KFMC), Riyadh, Saudi Arabia of patients from January 2015 to January 2021. A total of 340 patients were screened, of whom 140 were included in the analysis. Inclusion criteria included Saudi patients with stage IV or III NSCLC, aged between 40 to 85 years, with primary lung cancer and 1-year follow-up. Exclusion criteria included non-Saudi patients, SCLC, stages I and II of NSCLC, and metastatic lung cancer from other primary origins.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Institutional Review Board at King Fahad Medical City (No. IRB 21-088) and the Institutional Review Board at King Saud University (No. 21/0455/IRB). Written informed consent was waived due to the retrospective nature of the study, as only unidentifiable data were extracted from the medical records.
Statistical analyses
The electronic data of patients from 2015 to 2021 who met the inclusion criteria were retrieved from each center and an excel sheet was used to record the variables. The number of cases (%) was recorded for categorical variables and Fisher’s exact test or Pearson’s chi-square (χ2) test was used to compare them. Continuous variables were expressed as mean ± standard deviation (SD) and were compared using the independent Student’s t-test or one-way analysis of variance (ANOVA). The multivariate Cox-regression model was used to analyze the significant risk factor of death in stage IV lung cancer patients with a median survival time of 123 days.
A popular statistical tool in medical research to examine the relationship between a patient’s survival time and one or more predictor variables is the Cox proportional-hazards model (Cox, 1972). We used two R packages survival, survminer. We used R Survival and Survminer packages for survival analysis. For the stepwise selection, we used the function stepAIC in R to find out the best subset regression using Cox model that fits all covariates [Akaike Information Criterion (AIC) =427.08]. We did not encounter any major missing data. However, our data has variables with a small number of missing values and the distribution of the data was approximately normal. Therefore, imputation was minimally used in the process of filling in the missing values with the mean, median, or mode value of the non-missing values for that variable. P values were two-sided for all statistical tests, and a P value of 0.05 was considered statistically significant. Statistical analyses of the data were carried out, using the statistical package for the social sciences (SPSS Statistics for Windows, Version 20.0; IBM Corp., Armonk, NY, USA).
Results
A total of 140 patients with stage IV NSCLC were enrolled in this study. The mean age at diagnosis was 64 years old; 65.0% of the patients were male and smokers (37.9%). Demographic and clinical features of the whole cohort of NSCLC patients are presented in Table 1.
Table 1
| Variables | Description | N (%) |
|---|---|---|
| Gender | Male | 91 (65.0) |
| Female | 49 (35.0) | |
| Age (years) | Mean ± SD | 63.55±10.83 |
| BMI (kg/m2) | Mean ± SD | 26.28±7.45 |
| Stage | IV | 139 (99.3) |
| IIIB | 1 (0.7) | |
| Smoking | Smoker | 53 (37.9) |
| Ex-smoker | 14 (10.0) | |
| Never smoker | 43 (30.7) | |
| N/A | 30 (21.4) |
BMI, body mass index; SD, standard deviation; N/A, smoking data not available.
At presentation, metastasis of lymph nodes (58.6%), bone (40%), pleura (38.6%), and brain (35.7%) were the most common sites of disease associated with stage IV; managing the brain metastasis depended solely on radiation (37.9%) for most of the patients (Figure 1). Disease progression was higher for pleural metastasis compared to other metastatic sites (38.3%).
Four types of medical interventions were explored (Figure 2). Cell biomarker investigations for ALK and EGFR mutations tested positive in 10.7% of samples, while PD-L1 expression was higher (28.6%) (Table S1). Based on these results, the intent of treatment was mainly palliative care (90.7%), using first-line chemotherapy (61.4%) of pemetrexed, paclitaxel and carboplatin (Table S2). Immunotherapy (25.0%) was the second option of treatment, compared to anti-ALK (6.4%) and anti-EGFR (5.0%) medications (Table S3).
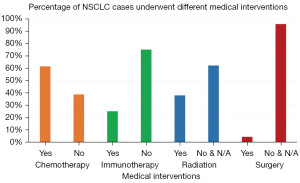
The results confirmed that advanced stages of NSCLC with metastatic disease patterns require systemic treatment options. Correlation between immunotherapy and chemotherapy revealed that disease progression was higher in the chemotherapy group (83%) compared to the immunotherapy group (29.8%). The standard treatment regimen for NSCLC was chemotherapy using pemetrexed and carboplatin; however, a statistically significant association of using paclitaxel when coupled with immunotherapy treatment using monoclonal antibodies was found, particularly for nivolumab and pembrolizumab (Table 2). Although statistically significant, further trials are needed to confirm if these results are clinically relevant.
Table 2
| Variables | Medications | Dose given | Immunotherapy | Hazard ratio | 95% CI | P value | |
|---|---|---|---|---|---|---|---|
| No (n=105) | Yes (n=35) | ||||||
| Chemotherapy | Paclitaxel | No | 42 (40.0) | 7 (20.0) | 0.9241 | 0.2248, 3.799 | 0.047* |
| Yes | 5 (4.8) | 0 | |||||
| Pemetrexed | No | 49 (46.7) | 13 (37.1) | 0.8529 | 0.4846, 1.501 | 0.101 | |
| Yes | 32 (30.5) | 21 (60.0) | |||||
| Carboplatin | No | 33 (31.4) | 8 (22.9) | 0.5225 | 0.298, 0.9162 | 0.518 | |
| Yes | 48 (45.7) | 27 (77.1) | |||||
| Cisplatin | No | 72 (68.6) | 28 (80.0) | 0.8049 | 0.3429, 1.889 | 0.588 | |
| Yes | 9 (8.6) | 6 (17.1) | |||||
| Immunotherapy | Pembrolizumab | No | 82 (78.1) | 4 (11.4) | 0.3112 | 0.1235, 0.7841 | <0.001* |
| Yes | 0 | 30 (85.7) | |||||
| Nivolumab | No | 81 (77.1) | 30 (85.7) | 1.418 | 0.4413, 4.555 | 0.013* | |
| Yes | 1 (1.0) | 5 (14.3) | |||||
Categorical data presented as frequency (%). *, P value is significant at P<0.05. CI, confidence interval.
The intent of treatment was mainly palliative care (90.7%). When stratified by response status to first-line treatment, there were no statistically significant differences in survival at 18 months among stable, regressed, and progressed patients. Only 70 patients (50%) had a known response status to first-line treatment, of whom 13 (9.3%) were stable, 10 (7.1%) regressed, and 47 (33.6%) progressed. Further analysis of patients’ response to first-line treatment revealed a significant improvement in treatment response if chemotherapy treatment was maintained without any reduction or interruption of dosage (Table 3).
Table 3
| Description | Response to first-line treatment | Hazard ratio | 95% CI | P value | ||
|---|---|---|---|---|---|---|
| Stable# (n=13) | Regression§ (n=10) | Progression† (n=47) | ||||
| Dose reduction | 2 (15.4) | 5 (50.0) | 5 (10.6) | 2.734 | 0.9726, 7.686 | 0.066 |
| Interruption due to toxicity | 0 | 0 | 15 (31.9) | – | – | 0.053 |
| Dose maintained | 5 (38.5) | 0 | 4 (8.5) | 1.961 | 0.4844, 7.942 | 0.042* |
| N/A | 6 (46.2) | 5 (50.0) | 23 (48.9) | 1.270 | 0.5330, 3.029 | 0.989 |
Categorical data presented as frequency (%). #, not fitting the criteria of neither progressive nor regressive disease; §, partial or complete disappearance of a tumor with/without cancer therapy; †, metastasis in the primary site (lung) alone or beyond the lungs to other sites; *, P value is significant at P<0.05. CI, confidence interval; N/A, dose information not available.
Discussion
In this comprehensive 6-year epidemiological study conducted between 2015 and 2021, we aimed to explore disease prognosis and therapeutic strategies in patients diagnosed with advanced NSCLC. The findings of this study could provide valuable insights into the long-term trends and outcomes of NSCLC patients, helping to inform and improve clinical management and treatment approaches for this challenging condition. We have examined various factors that may influence disease prognosis, including tumor characteristics, patient demographics, treatment modalities, and response rates. Additionally, we have investigated the effectiveness and safety of different therapeutic strategies employed during the study period, such as chemotherapy, targeted therapy, immunotherapy, and radiation therapy. This study is crucial for advancing our understanding of NSCLC and facilitating the development of more personalized and effective treatment options for patients facing this aggressive form of lung cancer. As a result of the disease’s complexity, successful treatment of lung cancer is dependent on several factors, including the patient characteristics of tumor histology, testing for biomarkers, and effective and timely communication between pathologists and oncologists (9). Therefore, reviewing and identifying the clinical findings of NSCLC cases associated with late-stage discovery will help in understanding the challenges, assist with the healthcare decision process for the future management of patients and improve our ability to control the disease.
The current study evaluated patients with advanced-stage NSCLC from 2015 to 2021 and analyzed their initial characteristics, patterns of diagnosis and treatment and outcomes. Our observational study demonstrated a large heterogeneity in both diagnostic procedures and treatment modalities amongst patients with advanced NSCLC in the actual setting in Riyadh, Saudi Arabia. We found that patterns of treatment were very different, with more than 13 different types of therapy being used as first-line therapy. All of these therapies are approved by the Food and Drug Administration (FDA) for advanced NSCLC by the Oncologic Drugs Advisory Committee. Medical interventions of our patients involved surgery, radiotherapy and chemotherapy, while a small number of patients underwent potentially curative surgical resection. In a European study on patients with NSCLC stage III, 23% of all patients had surgery, with or without any other treatment, where 37.1% of patients had been diagnosed with stage IIIA and 6.1% of patients with stage IIIB (10). This percentage is similar to the Canadian study by Moore et al., 2019, in which 21% of patients underwent surgery, either alone or as part of combined modality therapy (11). In addition, 80.3% of patients with stage III NSCLC received either systemic chemotherapy alone or in combination with other treatments. Radiotherapy was given to 11.8% of patients (10).
In a recent study, data from the Australian Lung Cancer Registry of 1,396 stage III NSCLC patients treated from 2012 to 2019 consecutively demonstrated that the number of surgeries decreased over time, while the number of people on continuous combined hormone replacement therapy (cCHRT) and immunotherapy increased (12). Poor outcomes were reported for patients who were eligible for neoadjuvant treatment but eventually converted to cCHRT, compared with those who immediately underwent chemo-radiation therapy (13). In our study, the standard treatment regimen for NSCLC was chemotherapy, using pemetrexed and carboplatin, although a statistically significant association of using paclitaxel was found when coupled with immunotherapy treatment using monoclonal antibodies, especially nivolumab and pembrolizumab. Further analysis of patients’ response to first-line treatment revealed a significant improvement in treatment response if chemotherapy treatment was maintained at the same dose without any reduction or interruption of dosage. Although our findings are statistically significant, further clinical trials are needed to confirm if they are clinically relevant. Previous studies have revealed that NSCLC patients who underwent triple therapy (combination of surgery with definitive chemoradiation), did not show significant improvement when compared to those on other therapies (14-16). In contrast, significant survival outcomes have been observed in the use of triple therapy regimens compared with chemoradiation alone as a first-line treatment (11,17). Further clinical trials are required to provide a scientific study intended to assess the efficacy and/or safety of applying any modifications to those therapeutic procedures.
It is important to investigate the risk factors associated with lung cancer mortality because the risk of mortality in patients with stage IV lung cancer was associated with being more than 64 years old, male and a smoker. The multivariate Cox-regression model in our study indicated that smoking was the significant risk factor for death in stage IV lung cancer patients with a median survival time of 123 days. In a previous study on stage I to III lung cancer, the overall survival analysis was statistically associated with patients’ race, histology and treatment modality (18). Nieva et al. in 2022 reviewed different treatment approaches and survival outcomes for patients with NSCLC between 2011–2018 and found that 28% of patients died before initiating second-line treatment after a median of 19.8 months of follow-up (19). This is consistent with previous findings that almost a third of patients die prior to receiving second-line therapy (20-22). This emphasizes the need for the discovery of innovative treatments to increase the overall survival of patients with NSCLC and improve progression-free survival in the context of first-line treatment. The prognosis for stage IV NSCLC is challenging, as the cancer has spread beyond the lungs to other parts of the body. The 5-year survival rate for stage IV NSCLC is typically lower compared to earlier stages of the disease. However, advancements in treatment options and personalized therapies have improved outcomes for some patients. It is crucial to consider that survival rates were reported in terms of median survival, which represents the midpoint in a range of outcomes. For stage IV NSCLC, the median survival rate is typically measured in months rather than years. However, it is important to remember that every patient’s situation is unique, and some individuals may respond differently to treatment and experience longer survival than the median. Staging of a tumour has been shown to be the strongest determinant risk factor of lung cancer survival. Many attempts have been made to establish an accurate prediction model for lung cancer prognosis by measuring the effect of a variety of risk factors, but tumour staging remains the most significant predictor of mortality (23).
In a study from the Korean Association of Lung Cancer Registry, the 5-year relative survival rate for stage IV NSCLC patients was reported at 10% (24). Subgroup analysis in the same study showed that the 5-year relative survival rate was higher among stage IV NSCLC patients than those without such genetic alterations. While survival rates for stage IV NSCLC are generally low, factors such as the presence of certain genetic alterations can significantly improve survival outcomes. However, it’s important to note that these survival rates can change with advances in therapy and personalized medicine approaches, underscoring the importance of regular updates on this topic. Chansky and colleagues conducted a retrospective analysis on 9,137 patients and showed a substantial correlation between survival and Tumour, Lymph Node, and Metastasis (TNM) staging, where the median survival for stage IIIA and IA patients was 19 and 95 months respectively (25). An analysis of lung cancer mortality predictors in Saudi Arabia between 2009–2013 found that three-quarters of patients were diagnosed with lung cancer at an advanced stage and the tumour stage was a strong predictor of mortality (2). In a European study on patients with stage III NSCLC, the survival rate was similar in patients with adenocarcinomas and squamous cell NSCLC (10). An observational study in Italy between 2012 and 2021 revealed that the 2- and 5-year survival rates for stage III NSCLC patients were 68% and 32% respectively, for stage IIIA 71% and 34%, and for stage IIIB–C 66% and 30% (26). Other than the stage of cancer, additional factors with a predictive effect include performance status (PS), age, gender (27), obesity (28), smoking history (29) and metastasis (2). Distant metastasis increased mortality by 6-fold in SCLC and by 3-fold in NSCLC, compared to localized tumors, confirming the impact of the diagnostic stage on survival (2). In our study, disease progression was higher with brain and pleural metastasis and was solely managed by radiation. The overall cure and survival rates for NSCLC remain low, particularly in metastatic disease. Hence, to achieve higher survival prediction accuracy, a more complex approach that integrates individual pathological markers and genetic factors is needed (30).
The study’s limitations should be acknowledged. Similar to other retrospective observational studies, some clinical information was either unavailable in the structured data or not routinely collected. For example, there was no screening tool implemented for smoking in both hospitals’ and patients’ files and information about passive smoking and the social and occupational history of the patients was missing. NSCLC was also usually discovered at a late stage of the cancer and over half of patients died within 1year of diagnosis. Therefore, the limited sample size might be attributed to the inclusion of only advanced stage IIIB and IV NSCLC.
Conclusions
We provided insights into the management and the survival outcomes of stages IIIB and IV NSCLC patients, highlighted the need for newer, more effective treatment options, and reinforced the evidence that earlier diagnosis of disease with targetable mutations provides a relevant benefit. Further large-scale prospective studies are urgently warranted to detect novel potentially predictive biomarkers and more specific and more potent inhibitors of specific signaling pathways and overcome the potential resistance mechanisms in patients progressing during therapy with a targeted molecule.
Acknowledgments
The authors thank the Research Center at King Fahad Medical City for their technical support. This work is dedicated to Dr. Ahmed AlBadr, the former leader of the Research Center, who is defeating lung cancer.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1816/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1816/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1816/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1816/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Institutional Review Board at King Fahad Medical City (No. IRB 21-088) and the Institutional Review Board at King Saud University (No. 21/0455/IRB). Written informed consent was waived due to the retrospective nature of the study, as only unidentifiable data were extracted from the medical records.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Alghamdi HI, Alshehri AF, Farhat GN. An overview of mortality & predictors of small-cell and non-small cell lung cancer among Saudi patients. J Epidemiol Glob Health 2018;7:S1-S6. [Crossref] [PubMed]
- Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008;83:355-67. [Crossref] [PubMed]
- Kenfield SA, Wei EK, Stampfer MJ, et al. Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control 2008;17:198-204. [Crossref] [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histo-logic characteristics and prognosis. Cancer 1995;75:2844-52. [Crossref] [PubMed]
- Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer 1995;75:191-202. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Walser T, Cui X, Yanagawa J, et al. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc 2008;5:811-5. [Crossref] [PubMed]
- Rothschild SI, Zippelius A, Eboulet EI, et al. SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial. J Clin Oncol 2021;39:2872-80. [Crossref] [PubMed]
- Zemanova M, Pirker R, Petruzelka L, et al. Care of patients with non-small-cell lung cancer stage III - the Central European real-world experience. Radiol Oncol 2020;54:209-20. [Crossref] [PubMed]
- Moore S, Leung B, Wu J, et al. Real-World Treatment of Stage III NSCLC: The Role of Trimodality Treatment in the Era of Immunotherapy. J Thorac Oncol 2019;14:1430-9. [Crossref] [PubMed]
- Woodford K, Koo K, Reynolds J, et al. Persisting Gaps in Optimal Care of Stage III Non-small Cell Lung Cancer: An Australian Patterns of Care Analysis. Oncologist 2023;28:e92-e102. [Crossref] [PubMed]
- Benet J, Toffart AC, Brichon PY, et al. Survival of clinical stage III NSCLC according to therapeutic strategy: Relevance of the tumor board decision in the era of immunotherapy. Cancer Treat Res Commun 2022;30:100508. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or with-out surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Eberhardt WE, Pöttgen C, Gauler TC, et al. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concur-rent Chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194-201. [Crossref] [PubMed]
- Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049-56. [Crossref] [PubMed]
- Dickhoff C, Hartemink KJ, van de Ven PM, et al. Trimodality therapy for stage IIIA non-small cell lung cancer: benchmarking multi-disciplinary team decision-making and function. Lung Cancer 2014;85:218-23. [Crossref] [PubMed]
- Karacz CM, Yan J, Zhu H, et al. Timing, Sites, and Correlates of Lung Cancer Recur-rence. Clin Lung Cancer 2020;21:127-135.e3. [Crossref] [PubMed]
- Nieva J, Reckamp KL, Potter D, et al. Retrospective Analysis of Real-World Manage-ment of EGFR-Mutated Advanced NSCLC, After First-Line EGFR-TKI Treatment: US Treatment Patterns, Attrition, and Survival Data. Drugs Real World Outcomes 2022;9:333-45. [Crossref] [PubMed]
- Chiang AC, Fernandes AW, Pavilack M, et al. EGFR mutation testing and treatment de-cisions in patients progressing on first- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer 2020;20:356. [Crossref] [PubMed]
- Nadler E, Pavilack M, Espirito JL, et al. Observational Study of Treatment Patterns in Patients with Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Non-Small Cell Lung Cancer After First-Line EGFR-Tyrosine Kinase Inhibitors. Adv Ther 2020;37:946-54. [Crossref] [PubMed]
- Shah R, Girard N, Nagar SP, et al. European and US Real-World Treatment Patterns in Patients with Epidermal Growth Factor Receptor Mutation-Positive Non-Small Cell Lung Cancer: A Retrospective Medical Record Review. Drugs Real World Outcomes 2021;8:537-45. [Crossref] [PubMed]
- Putila J, Remick SC, Guo NL. Combining clinical, pathological, and demographic fac-tors refines prognosis of lung cancer: a population-based study. PLoS One 2011;6:e17493. [Crossref] [PubMed]
- Jeon DS, Kim HC, Kim SH, et al. Five-Year Overall Survival and Prognostic Factors in Patients with Lung Cancer: Results from the Korean Association of Lung Cancer Regis-try (KALC-R) 2015. Cancer Res Treat 2023;55:103-11. [Crossref] [PubMed]
- Chansky K, Sculier JP, Crowley JJ, et al. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol 2009;4:792-801. [Crossref] [PubMed]
- Ferro A, Sepulcri M, Schiavon M, et al. The Multidisciplinary Approach in Stage III Non-Small Cell Lung Cancer over Ten Years: From Radiation Therapy Optimisation to Innovative Systemic Treatments. Cancers (Basel) 2022;14:5700. [Crossref] [PubMed]
- Sculier JP, Chansky K, Crowley JJ, et al. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol 2008;3:457-66.
- Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer 2017;104:52-7. [Crossref] [PubMed]
- Ou SH, Ziogas A, Zell JA. Prognostic factors for survival in extensive stage small cell lung cancer (ED-SCLC): the importance of smoking history, socioeconomic and marital statuses, and ethnicity. J Thorac Oncol 2009;4:37-43. [Crossref] [PubMed]
- Mahar AL, Compton C, McShane LM, et al. Refining Prognosis in Lung Cancer: A Re-port on the Quality and Relevance of Clinical Prognostic Tools. J Thorac Oncol 2015;10:1576-89. [Crossref] [PubMed]



