
Function of homocysteine and HE4 in endometrial carcinoma: verified by prospective experiment
Highlight box
Key findings
• Homocysteine (Hcy) is an important serological marker that can be used for the diagnosis of endometrial carcinoma (EC). When used in combination with human epididymal protein 4 (HE4), the diagnostic value is higher.
What is known and what is new?
• Hcy have been linked to several disorders, including cardiovascular and neurodegenerative diseases. However, research on the role of EC is limited.
• Hcy is a potential diagnostic marker for EC Combining it with HE4 in real-time further enhances the diagnostic accuracy.
What is the implication, and what should change now?
• Using Hcy can increase the diagnostic efficiency of EC.
Introduction
The incidence of endometrial carcinoma (EC) is increasing year by year. EC is the second most common cancer of the female reproductive system, especially in Asia (1,2). Existing research has shown that the prognosis of patients with EC worsens significantly as the disease progresses. The 5-year overall survival rates for localized, regional, and distant tumors are 91%, 57%, and 17%, respectively (3). Late diagnosis and untimely treatment are the important factors associated with a poor prognosis (4,5). Early detection is necessary to improve the prognosis of EC.
Diagnostic curettage and pathological examination are the diagnostic golden standards for EC (4,6) but diagnostic curettage and pathological examination are invasive procedures, and therefore not universally applicable, especially at basic medical institutions and economically underdeveloped regions. Diagnostic curettage and pathological examination of all patients suspected of EC are a significant challenge. Hence, screening for high-risk patients should be considered. Not all patients can be diagnosed promptly. Even for patients who undergo curettage, a missed curettage may affect the diagnosis (7,8). Imaging examinations by experts, such as ultrasonography and magnetic resonance imaging (MRI), are effective diagnostic aids, but experts are not ubiquitous available (9-11). In contrast, using a small-molecule marker for tumor diagnosis may offer a practical alternative that is noninvasive, simple, and reliable. The usefulness of small-molecule markers for EC diagnosis is worthy of exploration. Human epididymal protein 4 (HE4), carbohydrate antigen 125 (CA125) and carbohydrate antigen 199 (CA199) are widely used in the diagnosis of gynecological tumors (12-14). Despite this, the prognosis of EC has not been significantly improved (15,16). Hence, further investigations of small-molecule tumor markers are warranted.
Abnormal changes in the level of homocysteine (Hcy) among patients with EC have attracted our attention. Hcy, an intermediate product of methionine metabolism, is an amino acid-containing sulfhydryl (17,18). Folate is necessary for regulation Hcy and cell division (19). In humans, the rapid proliferation of cancer cells consumes excessive amounts of folate, which in turn, causing marked Hcy accumulation (20). The sulfhydryl of Hcy is responsible for the generation of superoxide and free radicals, causes vascular endothelial cell injury, and promoting cancer cell migration (21,22). The level of Hcy differs between benign endometrial diseases and other gynecological cancers, Hcy, which has been discovered by serum metabolomic analysis, is one of the most important metabolites in EC (23). Given the close relationship between Hcy and EC, we decided to investigate Hcy in patients with EC in this study.
Specifically, this study aimed to explore the role of Hcy in EC diagnosis and management. Given the limitations of individual small-molecule tumor markers, we included other common tumor markers, such as HE4, CA199, CA125, fibrinogen (Fib), and D-dimer (D-D) in our investigation. Furthermore, we examined whether simultaneous detection using multiple markers can improve diagnostic efficiency. We present this article in accordance with the STARD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1559/rc).
Methods
Patients
A total of 143 patients hospitalized for abnormal vaginal bleeding or discharge and diagnosed with EC at Nanjing Women and Children’s Healthcare Hospital from January 2016 to May 2019 were included in the pre-experiment. The diagnosis was confirmed by two or three independent pathologists. A control group of patients with benign gynecological diseases who visited Nanjing Women and Children’s Healthcare Hospital during the same period was included in this study. Clinical data were collected retrospectively. A verification experiment was performed between June 2019 and February 2020, and 118 patients were recruited. None of the patients recruited in this study had any other physical disorders (for example, other malignant tumors, thrombotic diseases, poor blood glucose or blood pressure control, endocrinological diseases, coagulation dysfunction, or liver and kidney dysfunction). This study was approved by the Medical Ethics Committee of Nanjing Women and Children’s Healthcare Hospital (No. NFKSL-003) and was registered at the Chinese Clinical Trial Registry (ChiCTR1900023149). This study is divided into two parts based on the time of pathological collection: pre-experiment and validation experiment. The preliminary experiment is a retrospective study and does not require participants to sign an informed consent form. On the other hand, the validation experiment is prospective and requires all participants to sign informed consent forms. All recruited patients for the verification experiment have provided the informed consent form. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Concentration measurement for candidate biomarker
Blood was sampled before the procedure and used for measuring the serum concentration of six small molecular markers. All measurements were performed at room temperature according to the manufacturers’ instructions and the investigators were blinded to the results of the histopathologic reports. A fully automatic chemistry analyzer 2700 (Olympus, Tokyo, Japan) was used to measure the level of Hcy. The COBAS 6000 analyzer (Roche, Basel, Switzerland) was used to measure the level of HE4, CA125, and CA199. CA7000 automated coagulation analyzer (Sysmex, Kobe, Japan) was used to measure the level of Fib and D-D.
Estimated sample of verification experiment
Each group included 59 patients (allocation ratio 1:1). A 20% attrition rate was taken into consideration. The sample size calculation considered 90% power to detect a difference of 0.183 between the area under the ROC under the null hypothesis of 0.5 and an area under the curve (AUC) under the alternative hypothesis of 0.683 (Hcy), using a two-sided z-test, at a significance level of 0.05.
Statistical analysis
SPSS version 23 (Chicago, Illinois, USA) and MedCalc Statistical Software version 15.6.1 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2015) were used for the statistical analyses. PASS 11 (NCSS, USA) was used to estimate the sample size for the validation experiments (24). Normally distributed data were presented as mean and standard deviation, and group comparisons were performed using Student’s t-test. Non-normally distributed data were presented as medians and interquartile ranges. Group comparisons were performed using the Mann-Whitney U test. ROC and Youden Index were used to assess diagnostic efficacy (25,26). Sensitivity (SN) and specificity (SP) at the ideal cutoffs were estimated based on the Youden Index (27). The areas under the ROC were compared using the DeLong method (28). New prediction methods were established through a review of binary metalogics, and the Hosmer-Lemeshow test was used to test the goodness of fit of a linear regression equation. P values <0.05 were considered statistically significant.
Results
All patients were recruited according to the process as shown in Figure 1. The basic clinical data of the included patients are presented in Table 1. The main difference between the pre-experiment and verification experiment was that the CA199 and CA125 between EG (experimental group) and CG (control group) were inconsistent.
Table 1
| Group | Pre-experiment | Verification experiment | |||||
|---|---|---|---|---|---|---|---|
| EG | CG | P | EG | CG | P | ||
| Number | 143 | 143 | – | 59 | 59 | – | |
| Age (year) | 53.518 (8.319) | 48.238 (9.214) | <0.001 | 56.051 (8.827) | 46.373 (8.428) | <0.001 | |
| BMI (kg/m2) | 24.088 (3.262) | 25.148 (3.922) | 0.014 | 25.897 (16.650) | 25.433 (3.664) | 0.209 | |
| Fib (g/L) | 2.51 (2.205–2.894) | 2.296 (2.00–2.627) | <0.001 | 2.852 (2.409–3.279) | 2.41 (2.10–2.69) | <0.001 | |
| D-D (mg/L) | 0.25 (0.18–0.40) | 0.20 (0.14–0.32) | 0.001 | 0.33 (0.19–0.49) | 0.205 (0.19–0.39) | 0.038 | |
| Hcy (μmol/L) | 8.80 (7.01–11.30) | 7.2 (6.20–8.50) | <0.001 | 7.80 (7.10–8.58) | 6.83 (6.10–8.00) | 0.001 | |
| HE4 (pmol/L) | 68.35 (51.84–102.90) | 52.96 (44.44–61.44) | <0.001 | 61.05 (46.16–80.80) | 42.39 (38.20–51.19) | <0.001 | |
| CA125 (U/L) | 18.65 (13.49–29.70) | 15.58 (11.46–23.79) | 0.017 | 19.44 (10.07–32.06) | 16.69 (12.84–24.90) | 0.865 | |
| CA199 (U/L) | 14.56 (8.43–27.72) | 9.22 (6.62–15.86) | <0.001 | 10.49 (7.08–19.43) | 9.75 (6.99–15.19) | 0.491 | |
| Composition of CG | |||||||
| Normal | – | 90 | – | – | 57 | <0.001* | |
| AEH | – | 53 | – | – | 2 | ||
| FIGO | |||||||
| I | 116 | – | – | 45 | – | 0.446* | |
| II–IV | 27 | – | – | 14 | – | ||
| Grade of differentiation | |||||||
| G1 | 66 | – | – | 29 | – | 0.695* | |
| G2–G3 | 64 | – | – | 23 | – | ||
| Undefined | 13 | – | 7 | ||||
Normally distributed data were presented as mean and standard deviation, non-normally distributed data were presented as medians and interquartile ranges. *, comparing the composition of subjects in the pre-experiment and validation experiment. EG, experimental group; CG, control group; BMI, body mass index; Fib, fibrinogen; D-D, D-dimer; Hcy, homocysteine; HE4, human epididymal protein 4; CA125, cancer antigen 125; CA199, cancer antigen 199; AEH, atypical hyperplasia of endometrium; FIGO, International Federation of Gynecology and Obstetrics; G, grade.
Univariate analysis showed that except for D-D and CA125, all markers were significantly associated with EC. Consequently, multivariate analysis was used to verify the results and confirmed that the relationship was indeed statistically significant (Table 2).
Table 2
| Variables | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| Relative risk | 95% CI | P value | Relative risk | 95% CI | P value | |||
| BMI | 1.087 | 1.016–1.163 | 0.015 | 1.109 | 1.022–1.203 | 0.013 | ||
| Hcy | 1.34 | 1.202–1.494 | <0.001 | 1.279 | 1.131–1.445 | <0.001 | ||
| Fib | 2.538 | 1.562–4.124 | <0.001 | 1.988 | 1.135–3.481 | 0.016 | ||
| D-D | 2.376 | 0.977–5.776 | 0.056 | – | ||||
| CA125 | 1.001 | 0.997–1.007 | 0.566 | – | ||||
| HE4 | 1.047 | 1.030–1.064 | <0.001 | 1.039 | 1.022–1.056 | <0.001 | ||
| CA199 | 1.038 | 1.017–1.059 | <0.001 | 1.029 | 1.006–1.052 | 0.012 | ||
CI, confidence interval; BMI, body mass index; Hcy, homocysteine; Fib, fibrinogen; D-D, D-dimer; CA125, cancer antigen 125; HE4, human epididymal protein 4; CA199, cancer antigen 199.
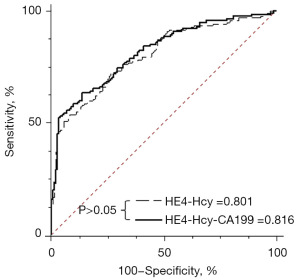
The area under the ROC curve for HE4 was the largest, indicating that HE4 had the best diagnostic performance for EC (Table 3). The diagnostic values of Hcy and CA199 were slightly lower than those of HE4, but the difference was not statistically significant (Table 3). Considering these results, different combinations of HE4, Hcy, and CA199 for the diagnosis of EC were investigated. The results showed that the combined detection of HE4, Hcy, and CA199 improved diagnostic efficiency. However, using the combination of HEA, Hcy, and CA199 for the diagnosis of EC was not significantly superior to using HE4 and Hcy (Figure 2). Considering cost-effectiveness, a diagnostic equation HE4-Hcy (H-H) was established by combining HE4 and Hcy (Figure 3). The model formulation of H-H was available because the P value of the Hosmer-Lemeshow test was larger than 0.05.
Table 3
| Characteristics | ROC-AUC | 95% CI | P* |
|---|---|---|---|
| HE4 | 0.747 | 0.692–0.796 | – |
| Hcy | 0.684 | 0.627–0.738 | 0.119 |
| CA199 | 0.671 | 0.613–0.725 | 0.059 |
| Fib | 0.620 | 0.561–0.677 | 0.003 |
| BMI | 0.580 | 0.520–0.639 | 0.001 |
*, other markers compared with HE4. ROC-AUC, area under the receiver operating characteristic curve; CI, confidence interval; HE4, human epididymal protein 4; Hcy, homocysteine; CA199, cancer antigen 199; Fib, fibrinogen; BMI, body mass index.
The coefficients in the prediction equation are as follows:
The predicted probability (PP) is:
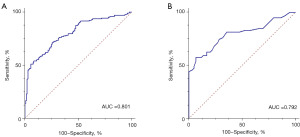
When H-H was used to diagnose EC, the area under ROC were 0.801 [95% confidence interval (CI): 0.750–0.846]. The Youden Index, SN, and SP were 0.455, 72.73%, and 72.73%, respectively. In the verification experiment, the area under ROC, Youden Index, SN, and SP, was 0.792 (95% CI: 0.708–0.861), 0.506, 57.63%, and 93.22%, respectively (Table 4).
Table 4
| Characteristics | Pre-experiment | Verification experiment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ROC-AUC (95% CI) | Youden Index | SN (%) | SP (%) | ROC-AUC (95% CI) | Youden Index | SN (%) | SP (%) | ||
| EG vs. CG | 0.801 (0.750–0.846) | 0.455 | 72.73 | 72.73 | 0.792 (0.708–0.861) | 0.506 | 57.63 | 93.22 | |
| EG vs. normal | 0.818 (0.763–0.865) | 0.504 | 72.73 | 77.66 | 0.797 (0.712–0.866) | 0.524 | 57.63 | 94.74 | |
| EG vs. AEH | 0.770 (0.704–0.827) | 0.438 | 60.14 | 83.67 | – | ||||
| FIGO | |||||||||
| I | 0.789 (0.734–0.837) | 0.426 | 69.83 | 72.73 | 0.762 (0.669–0.840) | 0.454 | 52.17 | 93.22 | |
| II–IV | 0.856 (0.794–0.905) | 0.647 | 81.48 | 83.22 | 0.898 (0.804–0.957) | 0.769 | 76.92 | 100.00 | |
| Age, years | |||||||||
| ≤50 | 0.871 (0.804–0.922) | 0.576 | 66.67 | 90.91 | 0.807 (0.683–0.898) | 0.557 | 81.25 | 74.42 | |
| >50 | 0.772 (0.695–0.837) | 0.441 | 64.13 | 80.00 | 0.762 (0.633–0.863) | 0.519 | 58.14 | 93.75 | |
| Grade of differentiation | |||||||||
| G1 | 0.761 (0.698–0.818) | 0.388 | 86.36 | 52.45 | 0.675 (0.557–0.779) | 0.332 | 68.75 | 64.41 | |
| G2–3 | 0.851 (0.788–0.901) | 0.628 | 85.19 | 77.62 | 0.919 (0.827–0.971) | 0.663 | 90.00 | 76.27 | |
SN, sensitivity; SP, specificity; EC, endometrial cancer; ROC-AUC, the area under the receiver operating characteristic curve; CI, confidence interval; EG, experimental group; CG, control group; AEH, atypical hyperplasia of endometrium; FIGO, International Federation of Gynecology and Obstetrics; G, grade.
Subgroup analysis was performed according to patient characteristics (Table 4). The results showed that the diagnostic value of H-H was most applicable to patients with EC stage II–IV, those older than 50 years, or in cases where the G2–3 differentiation was especially prominent. Importantly, the results of the pre- and verification experiments were consistent.
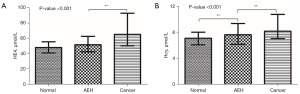
Broadly, there are two stages of EC development, namely, atypical hyperplasia of endometrium (AEH) and endometrial cancer. Hence, if we can arrest EC progression by timely diagnosis and early treatment of atypical endometrial hyperplasia, the conditions of the patients would be greatly improved. Subsequently, we divided the participants into three groups for further analysis: (I) women with no endometrial lesions; (II) women with AEH; and (III) women with EC. The results showed that only Hcy level differed significantly among the three groups (Figure 4).
Discussion
EC is one of the most common tumors of the female reproductive system, particularly in menopausal women. The most common symptoms are abnormal uterine bleeding and/or vaginal discharge. Postmenopausal women with abnormal uterine bleeding or vaginal discharge should be paid attention to. However, an existing meta-analysis indicated that only 9% (95% CI: 8–11%) of postmenopausal women were diagnosed with EC (29). Thus, all women with abnormal uterine bleeding or vaginal discharge should undergo diagnostic curettage despite medical resource wastage. The 5-year overall survival rate of patients is closely related to disease progression, with distant metastasis at 17% (3). Hence, new reliable clinical EC detection markers are urgently needed, especially for the detection of EC before distant metastasis.
There are currently no blood biomarkers for routine clinical use in EC. HE4 is a glycoprotein that is overexpressed in the serum of patients with EC, making it a good candidate for use as a diagnostic biomarker (6,30) Some studies reported that when HE4 was used for the diagnosis of EC, AUC of the analysis fluctuated between 0.76 to 0.97, implying its potential as a promising non-invasive biomarker. However, HE4 levels rise with age and renal dysfunction, which may affect the interpretation of results (2,31). Therefore, the combined use of multiple markers can further enhance the application value.
Hcy is an important serum tumor marker for qualitative diagnosis of disease. However, there is limited research on its role in the diagnosis of EC. Therefore, we conducted research on this topic. The area under ROC was greater than 0.5, indicating that Hcy could be used for the diagnosis of EC. Moreover, Hcy progressively increases as the disease progresses. Consequently, by detecting the change in Hcy level, especially in patients with abnormal uterine bleeding and/or vaginal discharge, patients who are highly suspected of EC may be distinguished from those who do not actually require diagnostic curettage, leading to a timely and definitive diagnosis. In comparing Hcy with the most commonly used EC diagnostic markers, HE4 and CA125, the Hcy ROC-AUC was not significantly different from that of HE4, and significantly larger than that of CA125. Hence, Hcy is a potentially useful and noteworthy diagnostic marker for EC.
D-D and Fib levels are often used to reveal changes in blood coagulation functions. The coagulation pathway in endothelial cells is activated by tumor cells, leading to the secretion of procoagulants, resulting in a secondary increase in fibrinolysis and D-D, which are the degradation products of fibrin (32,33). The relationship between changes in D-D and Fib levels and EC prognosis has been previously reported (34-36). To date, the role of D-D and Fib in EC diagnosis has been overlooked. The results of this study suggest that changes in D-D and Fib levels could be used to predict EC. CA199 was also included in this study and our results confirmed its application in predicting EC, which is consistent with previous study (37).
Many markers for EC diagnosis have been suggested but their performance has not been satisfactory. Until more efficient diagnostic markers become available, we must compensate for this deficiency. The use of multiple tumor markers for EC diagnosis may offer a promising alternative. It is recognized that the use of a large number of biomarkers can improve diagnosis, but it also leads to medical resource wastage, and adds to the patients’ financial burden. Choosing appropriate markers to establish a diagnostic model may offer a significant diagnostic alternative. Our study showed that a combination of HE4, Hcy, and CA199 could improve the diagnostic accuracy of EC. However, the improvement was not significantly greater than that observed with HE4 and Hcy. Considering the medical costs, HE4 and Hcy were selected for the diagnostic model and verified in an independent population.
Our findings demonstrated that Hcy can be used in the diagnosis of EC and for assessing the disease status. Moreover, Fib and D-D can also be utilized to predict EC, although they may be diagnostically less valuable than Hcy. Importantly, using the combination of HE4, Hcy, CA199, and Fib together for EC diagnosis is more effective than utilizing each of these markers independently. In conclusion, by combining HE4, Hcy, CA199, and Fib detection, diagnostic accuracy can be significantly improved.
Study limitations
This study is a single-center study with a small sample size. Before clinical implementation, further verification through multi-center large-sample experiments is needed.
Conclusions
This study confirms that Hcy can be used as a diagnostic tool for EC. Additionally, the combination of Hcy with HE4 enhances its application value. Although this improvement may not have a significant impact on the patient’s prognosis, it provides valuable insights for future research, implying that common biomarkers may have a greater impact in new areas.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1559/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1559/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1559/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1559/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eslahi M, Roshandel G, Torkian S. Trends in the Incidence Rates of Breast and Gynecological Cancers in Asia from 1998-2012: An Ecological Study. Arch Iran Med 2022;25:112-7. [Crossref] [PubMed]
- Hertlein L, Stieber P, Kirschenhofer A, et al. Human epididymis protein 4 (HE4) in benign and malignant diseases. Clin Chem Lab Med 2012;50:2181-8. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Njoku K, Barr CE, Crosbie EJ. Current and Emerging Prognostic Biomarkers in Endometrial Cancer. Front Oncol 2022;12:890908. [Crossref] [PubMed]
- Crosbie EJ, Kitson SJ, McAlpine JN, et al. Endometrial cancer. Lancet 2022;399:1412-28. [Crossref] [PubMed]
- Barr CE, Njoku K, Jones ER, et al. Serum CA125 and HE4 as Biomarkers for the Detection of Endometrial Cancer and Associated High-Risk Features. Diagnostics (Basel) 2022;12:2834. [Crossref] [PubMed]
- Deckardt R, Lueken RP, Gallinat A, et al. Comparison of transvaginal ultrasound, hysteroscopy, and dilatation and curettage in the diagnosis of abnormal vaginal bleeding and intrauterine pathology in perimenopausal and postmenopausal women. J Am Assoc Gynecol Laparosc 2002;9:277-82. [Crossref] [PubMed]
- Liberis A, Mareti E, Pratilas G, et al. Dilatation and curettage in endometrial cancer. What is the correlation with hysterectomy histology? A 14 years retrospective cohort study. J BUON 2021;26:1775-81. [PubMed]
- Lefebvre TL, Ueno Y, Dohan A, et al. Development and Validation of Multiparametric MRI-based Radiomics Models for Preoperative Risk Stratification of Endometrial Cancer. Radiology 2022;305:375-86. [Crossref] [PubMed]
- Keles DK, Evrimler S, Merd N, et al. Endometrial cancer: the role of MRI quantitative assessment in preoperative staging and risk stratification. Acta Radiol 2022;63:1126-33. [Crossref] [PubMed]
- Gentry-Maharaj A, Karpinskyj C. Current and future approaches to screening for endometrial cancer. Best Pract Res Clin Obstet Gynaecol 2020;65:79-97. [Crossref] [PubMed]
- Ge L, Liu G, Hu K, et al. A New Risk Index Combining d-Dimer, Fibrinogen, HE4, and CA199 Differentiates Suspecting Endometrial Cancer From Patients With Abnormal Vaginal Bleeding or Discharge. Technol Cancer Res Treat 2020;19:1533033819901117. [Crossref] [PubMed]
- Prueksaritanond N, Angsathapon S, Insin P. The Utility of Preoperative Serum CA125 Combined with HE4 to Predict Lymph Node Metastasis in Endometrial Cancer. Gynecol Obstet Invest 2023;88:53-60. [Crossref] [PubMed]
- Lombaers MS, Cornel KMC, Visser NCM, et al. Preoperative CA125 Significantly Improves Risk Stratification in High-Grade Endometrial Cancer. Cancers (Basel) 2023;15:2605. [Crossref] [PubMed]
- Zheng RS, Zhang SW, Sun KX, et al. Cancer statistics in China, 2016. Zhonghua Zhong Liu Za Zhi 2023;45:212-20. [PubMed]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Hasan T, Arora R, Bansal AK, et al. Disturbed homocysteine metabolism is associated with cancer. Exp Mol Med 2019;51:1-13. [Crossref] [PubMed]
- Liu Z, Cui C, Wang X, et al. Plasma Levels of Homocysteine and the Occurrence and Progression of Rectal Cancer. Med Sci Monit 2018;24:1776-83. [Crossref] [PubMed]
- Al Mutairi F. Hyperhomocysteinemia: Clinical Insights. J Cent Nerv Syst Dis 2020;12:1179573520962230. [Crossref] [PubMed]
- Hoffman RM, Yano S. Tumor-Specific S/G(2)-Phase Cell Cycle Arrest of Cancer Cells by Methionine Restriction. Methods Mol Biol 2019;1866:49-60. [Crossref] [PubMed]
- Bukhari SA, Zafar K, Rajoka M, et al. Oxidative stress-induced DNA damage and homocysteine accumulation may beinvolved in ovarian cancer progression in both young and old patients. Turk J Med Sci 2016;46:583-9. [Crossref] [PubMed]
- McCully KS. Homocysteine, Thioretinaco Ozonide, and Oxidative Phosphorylation in Cancer and Aging: A Proposed Clinical Trial Protocol. Methods Mol Biol 2019;1866:285-310. [Crossref] [PubMed]
- Troisi J, Sarno L, Landolfi A, et al. Metabolomic Signature of Endometrial Cancer. J Proteome Res 2018;17:804-12. [Crossref] [PubMed]
- Wang YY, Sun RH. Application of PASS in sample size estimation of non-inferiority, equivalence and superiority design in clinical trials. Zhonghua Liu Xing Bing Xue Za Zhi 2016;37:741-4. [PubMed]
- Zhou Q, Hu J, Li X, et al. Comparison of traditional, trimmed traditional and robust Youden charts. Clin Chim Acta 2015;446:213-7. [Crossref] [PubMed]
- Yin J, Tian L. Joint confidence region estimation for area under ROC curve and Youden index. Stat Med 2014;33:985-1000. [Crossref] [PubMed]
- Martínez-Camblor P, Pardo-Fernández JC. The Youden Index in the Generalized Receiver Operating Characteristic Curve Context. Int J Biostat 2019; [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. [Crossref] [PubMed]
- Matteson KA, Robison K, Jacoby VL. Opportunities for Early Detection of Endometrial Cancer in Women With Postmenopausal Bleeding. JAMA Intern Med 2018;178:1222-3. [Crossref] [PubMed]
- Behrouzi R, Barr CE, Crosbie EJ. HE4 as a Biomarker for Endometrial Cancer. Cancers (Basel) 2021;13:4764. [Crossref] [PubMed]
- Omer B, Genc S, Takmaz O, et al. The diagnostic role of human epididymis protein 4 and serum amyloid-A in early-stage endometrial cancer patients. Tumour Biol 2013;34:2645-50. [Crossref] [PubMed]
- Plantureux L, Crescence L, Dignat-George F, et al. Effects of platelets on cancer progression. Thromb Res 2018;164:S40-7. [Crossref] [PubMed]
- Ma M, Cao R, Wang W, et al. The D-dimer level predicts the prognosis in patients with lung cancer: a systematic review and meta-analysis. J Cardiothorac Surg 2021;16:243. [Crossref] [PubMed]
- Li Q, Cong R, Kong F, et al. Fibrinogen Is A Coagulation Marker Associated With The Prognosis Of Endometrial Cancer. Onco Targets Ther 2019;12:9947-56. [Crossref] [PubMed]
- Xu L, He F, Wang H, et al. A high plasma D-dimer level predicts poor prognosis in gynecological tumors in East Asia area: a systematic review and meta-analysis. Oncotarget 2017;8:51551-8. [Crossref] [PubMed]
- Nakamura K, Nakayama K, Ishikawa M, et al. High pretreatment plasma D-dimer levels are related to shorter overall survival in endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol 2016;201:89-93. [Crossref] [PubMed]
- Łaniewski P, Cui H, Mahnert ND, et al. Protein biomarkers in cervicovaginal lavages for detection of endometrial cancer. Biomark Res 2022;10:88. [Crossref] [PubMed]



