
Multivariate logistic regression analysis of the clinical factors influencing locally advanced prostate cancer
Highlight box
Key findings
• Higher prostate-specific antigen (PSA) and lower body mass index (BMI) indicating higher T stage and poorer prognosis in locally advanced prostate cancer.
What is known and what is new?
• It is known that a higher PSA value or a higher BMI means a higher likelihood of developing prostate cancer.
• The association of BMI with the progression of disease has not been reported in locally advanced prostate cancer, and our combined analysis with PSA is unprecedented.
What is the implication, and what should change now?
• When we encounter prostate cancer patients in clinical practice with higher PSA levels and significant BMI reduction, this may indicate a later stage of prostate cancer and a worse prognosis. It can help us to focus more on the selection of preoperative and postoperative treatment plans rather than solely on surgical treatment.
Introduction
Cancer of the prostate gland [prostate cancer (PCa)] is the second most commonly diagnosed cancer in men, with an estimated 1.4 million diagnoses made worldwide in 2020 (1). A systematic review of autopsy studies reported a prevalence of PCa at age <30 years of 5% (95% confidence interval: 3–8%), increasing by an odds ratio of 1.7 (1.6–1.8) per decade, to a prevalence of 59% (48–71%) by age >79 years (2). There is variation in the prevalence of autopsy-detected PCa between men with different ethnic backgrounds and geographical areas (e.g., 83 in white US males vs. 41 in Japan at age 71–80 years) (3).
In China, the proportion of patients with locally advanced PCa is significantly higher than that in developed countries. locally advanced PCa treatment usually involves anti-androgen therapy, chemotherapy, surgery, and combination therapy, of which radical prostatectomy is the most efficacious (4). However, the prognosis of patients is usually poor because of the high risk of metastasis. Hence, exploring the factors associated with locally advanced PCa may point to a new direction in its treatment.
Locally advanced cancer is referring to patients with T3 or T4 cancers and no nodal or distant metastatic disease. Lee and colleagues found that the T stage was significantly relevant to positive surgical margins (PSMs), which led to a poor prognosis (5). This observation showed the close connection between T staging and the prognosis of PCa. It has been reported that the T stage, serum level of prostate-specific antigen (PSA), and Gleason score are independent factors of a poor prognosis of PCa (6,7), but the relevant factors to T staging are not known.
Body mass index (BMI) is associated with several types of cancer (8). Tzenios and colleague found that a greater BMI is linked to a higher risk of PCa (9), adipose stromal cells (ASCs) are crucial drivers of aggressiveness in patients with obesity and PCa. A variety of mechanisms through which ASCs modulate the tumour microenvironment (TME) by secreting various adipokines have been proposed to account for the role of white adipose tissue (WAT) in obesity-driven PCa progression (10). Zorena’s research showed that BMI is associated with PCa-related mortality, possibly due to a systemic inflammatory response to high BMI (11). Zhang and colleagues reported a negative association between BMI and the PSA level in patients with PCa (12). Those findings suggest that BMI may be an important factor in PCa progression, but whether it affects locally advanced PCa is not known.
The PSA level plays an important part in the screening, diagnosis, postoperative monitoring, and disease assessment of PCa. We investigated the correlation between the pathologic T stage and clinical indicators [BMI, PSA, Gleason score, neutrophil-lymphocyte ratio (NLR), lymphocyte-monocyte ratio (LMR)] (13). We explored the risk factors for locally advanced PCa to provide a reference for the diagnosis and treatment of PCa. And we presented this article in accordance with the STARD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1680/rc).
Methods
Ethical approval of the study protocol
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research protocol was approved (No. 2023-RE-189) by the Ethics Review Committee of the First Affiliated Hospital (FAH) of the University of Science and Technology of China (USTC; Hefei, China).
Study population
From 1 January 2015 to 1 May 2020, we collected all the data of 177 patients diagnosed with PCa by preoperative imaging or biopsy who underwent abdominal surgery in the Department of Urology of the FAH within USTC. The data of patients undergoing Laparoscopic radical prostatectomy or robot-assisted radical prostatectomy were obtained. Postoperative specimens were confirmed to be PCa by at least three uropathologists from the FAH of USTC. All patients’ clinical data were exempted from informed consent under the supervision of the Ethics Review Committee of the First Affiliated Hospital of the USTC and their clinical data were used in a de-identified format for our study.
Inclusion and exclusion criteria
Data on BMI, PSA level, Gleason score, hypertension, N stage, M stage, and pathologic T stage were collected. TNM staging (pathologic T stage N stage and M stage) was determined based on imaging and pathology by two experts who have been engaged in urology for more than 10 years. Patients with missing clinical data were not included in the study cohort. The relationship between these indicators and the pathologic T stage was explored (9).
The inclusion criteria were patients: (I) who underwent radical surgery for PCa at the First Affiliated Hospital of the University of Science and Technology of China; (II) whose postoperative pathology clearly showed PCa; (III) aged <75 years.
The exclusion criteria were patients: (I) with acute infectious diseases; (II) with hematological diseases that may cause alterations in the neutrophil count in peripheral blood; (III) with bladder stones; (IV) with acute urinary retention; (V) who had undergone prostate gland- or urethra-related surgery within the previous two weeks; (VI) who had regular drug (e.g., finasteride) interventions; (VII) with other tumors; (VIII) with incomplete clinical data.
Statistical analysis
Statistical analyses were carried out using SPSS 19.0 (IBM, Armonk, NY, USA). Data with the variables conforming to the normal distribution were summarized as mean ± standard deviation. Data with the variables departing from the normal distribution were summarized as medians and interquartile intervals. Data with a normal distribution were analyzed by ANOVA (analysis of variance) for differences between groups. Data with a non-normal distribution were compared by the Kruskal-Wallis test. The Chi-square test was used for comparison of prevalence. Data with a non-normal distribution were analyzed by Spearman correlation analysis to find factors associated with the pathologic T stage, and by logistic ordered multiple regression analysis to find independent risk factors. Receiver operating characteristic (ROC) curves were used to assess the diagnostic accuracy of the T stage of PCa. In the analysis of ROC curves, T3 and T4 were combined and classified as a “locally progressive” population, and T2 as a “locally non-progressive” population. P<0.05 was considered significant.
Results
Analysis of the variability of clinical indicators in patients with different T stages of PCa
Of the 177 patients with PCa disease, there were 142 in stage T2, while there were 18 and 17 patients in stages T3 and T4, respectively. Data with a non-normal distribution data were shown as the median (25–75%) (Table 1). ANOVA revealed a significant difference in BMI between different T stages. Two-by-two ANOVA showed BMI to be significantly lower in patients with T4 stage than in those with T2 stage (P<0.001) (Table 1; Figure 1A), but differences between the other stages were not significant. Two-by-two ANOVA showed that the PSA level in patients with T2 or T3 stages were significantly different (P<0.05) (Table 1; Figure 1B), but differences in the PSA level between patients with other stages were not significant. The Chi-square test showed significant differences in the prevalence of hypertension (P=0.024), Gleason score (P=0.025), N stage (P=0.018), and M stage (P=0.001) among T stages (Table 1), but significant differences were not found between the T stages for the systemic inflammatory indicators NLR or platelet to lymphocyte ratio (PLR).
Table 1
| Variables | All patients, n=177 | T2, n=142 | T3, n=18 | T4, n=17 | P |
|---|---|---|---|---|---|
| Age (years) | 69 (65–75) | 69.00 (65.00–75.00) | 70.72±5.09 | 69.94±6.88 | 0.82 |
| BMI (kg/m2) | 23.27±3.24 | 23.69±3.05 | 22.43±3.40 | 20.63±3.15 | <0.001 |
| WBC count (109/L) | 5.96±1.31 | 5.97±1.31 | 6.23±1.26 | 5.59±1.39 | 0.339 |
| Neu (%) | 60.84±7.53 | 61.09±7.43 | 60.46±8.93 | 58.60 (58.00–62.50) | 0.615 |
| L (%) | 29.00±7.13 | 28.63±7.08 | 30.04±7.91 | 31.00±6.62 | 0.351 |
| Mon (%) | 7.00 (5.45–8.40) | 6.90 (5.48–8.50) | 7.14±1.57 | 6.82±1.80 | 0.849 |
| PLT count (109/L) | 189.45±62.74 | 183.00 (140.50–223.00) | 205.61±74.57 | 185.24±65.30 | 0.606 |
| Neu count (109/L) | 3.64±0.99 | 3.66±0.98 | 3.78±1.06 | 3.32±0.99 | 0.324 |
| L count (109/L) | 1.72±0.57 | 1.62 (1.32–1.98) | 1.86±0.60 | 1.73±0.61 | 0.54 |
| Mon count (109/L) | 0.42±0.14 | 0.42±0.14 | 0.44±0.10 | 0.37±0.09 | 0.273 |
| NLR | 2.07 (1.67–2.82) | 2.07 (1.74–2.91) | 2.24±0.99 | 2.03±0.65 | 0.443 |
| LMR | 3.93 (3.15–5.50) | 3.84 (3.03–5.98) | 4.43±1.71 | 4.34 (3.58–5.17) | 0.456 |
| NMR | 8.71 (7.03–11.20) | 8.79 (7.01–11.27) | 8.89±2.258 | 8.01 (7.48–11.47) | 0.819 |
| PWR | 30.45 (24.72–37.31) | 30.43 (24.92–37.13) | 33.26±10.85 | 30.99±11.04 | 0.911 |
| PSA (ng/mL) | 32.36 (14.77–94.60) | 28.47 (14.11–61.28) | 146.48 (39.35–202.85) | 176.36±356.50 | 0.006 |
| PSA subgroup (ng/mL) | <0.001 | ||||
| <10 | 15 | 15 | 0 | 0 | |
| 10 to <20 | 50 | 49 | 1 | 0 | |
| 20 to <50 | 52 | 43 | 4 | 5 | |
| 50 to <100 | 17 | 12 | 2 | 3 | |
| ≥100 | 43 | 23 | 11 | 9 | |
| Hypertension | 0.024 | ||||
| Yes | 41 (23.2) | 39 | 1 | 1 | |
| No | 136 (76.8) | 103 | 17 | 16 | |
| Diabetes | 0.43 | ||||
| Yes | 13 (7.3) | 12 | 1 | 0 | |
| No | 164 (92.7) | 130 | 17 | 17 | |
| Gleason score | 0.025 | ||||
| 6 | 25 (14.1) | 24 | 0 | 1 | |
| 7 | 80 (45.2) | 65 | 10 | 5 | |
| 8 | 32 (18.1) | 26 | 4 | 2 | |
| 9 | 35 (19.8) | 23 | 3 | 9 | |
| 10 | 5 (2.8) | 4 | 1 | 0 | |
| N stage | 0.018 | ||||
| N0 | 168 (94.9) | 138 | 15 | 15 | |
| N1 | 9 (5.1) | 4 | 3 | 2 | |
| M stage | 0.001 | ||||
| M0 | 144 (81.4) | 123 | 12 | 9 | |
| M1 | 33 (18.6) | 19 | 6 | 8 |
Data conforming to the normal distribution were summarized as mean ± standard deviation and analyzed by ANOVA (analysis of variance) for differences among groups. Data departing from the normal distribution were summarized as medians and interquartile intervals and compared by the Kruskal-Wallis test. Data are presented as n (%) or n unless otherwise stated. BMI, body mass index; WBC, white blood cell; Neu, neutrophil; L, lymphocyte; Mon, monocyte; PLT, platelet; NLR, neutrophil-lymphocyte ratio; PWR, platelet-white blood cell ratio; LMR, lymphocyte-monocyte ratio; NMR, neutrophil-monocyte ratio; PSA, prostate specific antigen; N, lymph node; N0, regional (pelvic) lymph node negative; N1, regional (pelvic) lymph node positive; M, Metastasis; M0, metastasis negative; M1, metastasis positive.
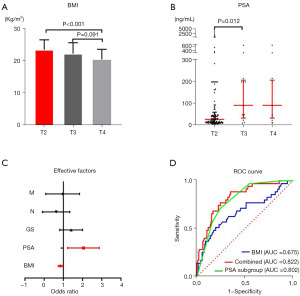
Spearman’s rank correlation and factors associated with T-stage
The results of Spearman’s rank correlation and clinical indicators related to the T stage in patients with PCa showed that the PSA level (r=0.427, P=0.003), M stage (r=0.279, P<0.001), Gleason score (r=0.197, P=0.009), and N stage (r=0.201, P=0.007) showed a significant positive correlation with the T stage. Interestingly, there was a negative correlation between BMI (r=−0.255, P=0.001) and T-stage (Table 2), suggesting that a higher T stage was associated with lower BMI in patients suffering from PCa. Hypertension (r=−0.204, P=0.006) also showed a negative association with the T stage, but a correlation was not found between the other clinical indicators we tested and the T stage.
Table 2
| Variables | R | P |
|---|---|---|
| Age (years) | 0.019 | 0.802 |
| BMI (kg/m2) | −0.255 | 0.001 |
| WBC (109/L) | −0.016 | 0.834 |
| Neu (%) | −0.064 | 0.395 |
| L (%) | 0.099 | 0.19 |
| Mon (%) | −0.016 | 0.835 |
| Neu (109/L) | −0.066 | 0.382 |
| L (109/L) | 0.052 | 0.495 |
| Mon (109/L) | −0.038 | 0.613 |
| PLT (109/L) | 0.037 | 0.629 |
| NLR | −0.094 | 0.216 |
| PWR | 0.023 | 0.763 |
| NMR | −0.035 | 0.644 |
| LMR | 0.079 | 0.296 |
| PSA (ng/mL) | 0.427 | 0.003 |
| Gleason score | 0.197 | 0.009 |
| Hypertension | −0.204 | 0.006 |
| Diabetes | −0.09 | 0.233 |
| N stage | 0.201 | 0.007 |
| M stage | 0.279 | <0.001 |
Spearman correlation analysis was performed to find factors associated with the pathologic T stage. BMI, body mass index; WBC, white blood cell; Neu, neutrophil; L, lymphocyte; Mon, monocyte; PLT, platelet; NLR, neutrophil-lymphocyte ratio; PWR, platelet-white blood cell ratio; LMR, lymphocyte-monocyte ratio; PSA, prostate specific antigen; N, lymph node; M, metastasis.
Univariate and multivariate ordinal logistic analyses for screening the independent predictors of clinical T stages
Univariate logistic analyses showed that BMI, PSA level, Gleason score, hypertension, N stage, and M stage were risk factors for the T stage in patients with PCa. A multifactorial analysis of these clinical indicators showed that BMI and the PSA level were independent risk factors (Table 3; Figure 1C), and that the Gleason score, hypertension, N stage, and M stage were not independent risk factors, for the T stage in patients with PCa. The area under the ROC curve (AUC) showed that the PSA level (AUC =0.802) was more relevant than BMI (AUC =0.675) for diagnosing the T stage in patients suffering from PCa. Also, the sensitivity of the PSA level was higher than that of BMI (71.4% vs. 51.4%), whereas the specificity of BMI was higher than that of the PSA level (80.3% vs. 75.4%), for diagnosing the T-stage in patients suffering from PCa. A combination of BMI and the PSA level increased the accuracy (AUC =0.822) (Figure 1D; Table 4) and improved the sensitivity (88.6%, Table 4), but decreased the specificity (64.8%, Table 4), of the diagnosis.
Table 3
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | B | OR | 95% CI | P | ||
| Age (years) | 1.006 | (0.950–1.065) | 0.838 | |||||
| BMI (kg/m2) | 0.797 | (0.703–0.903) | <0.001 | −0.172 | 0.842 | (0.733–0.967) | 0.014 | |
| WBC (109/L) | 0.95 | (0.717–1.259) | 0.721 | |||||
| Neu (%) | 0.977 | (0.931–1.026) | 0.366 | |||||
| L (%) | 1.038 | (0.985–1.093) | 0.16 | |||||
| Mon (%) | 0.968 | (0.802–1.169) | 0.737 | |||||
| Neu (109/L) | 0.873 | (0.596–1.278) | 0.483 | |||||
| L (109/L) | 1.31 | (0.699–2.455) | 0.4 | |||||
| Mon (109/L) | 0.32 | (0.019–5.344) | 0.428 | |||||
| PLT (109/L) | 1.002 | (0.996–1.007) | 0.589 | |||||
| NLR | 0.754 | (0.481–1.179) | 0.159 | |||||
| PWR | 0.998 | (0.964–1.035) | 0.922 | |||||
| NMR | 0.97 | (0.858–1.097) | 0.632 | |||||
| LMR | 1.053 | (0.873–1.273) | 0.588 | |||||
| PSA (ng/mL) | 2.56 | (1.786–3.669) | <0.001 | 0.661 | 1.937 | (1.284–2.921) | 0.002 | |
| Gleason score | 1.581 | (1.117–2.234) | 0.01 | 0.278 | 1.32 | (0.856–2.038) | 0.209 | |
| Hypertension | 0.161 | (0.037–0.700) | 0.015 | −1.51 | 0.221 | (0.046–1.069) | 0.061 | |
| Diabetes | 0.306 | (0.037–2.509) | 0.3 | |||||
| N stage | 1.241 | (1.061–2.199) | 0.02 | −1.044 | 0.352 | (0.086–1.438) | 0.146 | |
| M stage | 1.25 | (1.104–1.650) | <0.001 | −0.362 | 0.696 | (0.249–1.946) | 0.49 | |
Logistic ordered multiple regression analysis to find independent risk factors. OR, odds ratio; CI, confidence interval; BMI, body mass index; WBC, white blood cell; Neu, neutrophil; L, lymphocyte; Mon, monocyte; PLT, platelet; NLR, neutrophil-lymphocyte ratio; PWR, platelet-white blood cell ratio; LMR, lymphocyte-monocyte ratio; PSA, prostate specific antigen; N, lymph node; M, metastasis.
Table 4
| Variables | AUC | 95% CI | Sensitivity | Specificity | P |
|---|---|---|---|---|---|
| PSA | 0.802 | (0.730–0.873) | 71.40% | 75.40% | <0.001 |
| BMI | 0.675 | (0.505–0.713) | 51.40% | 80.30% | 0.046 |
| PSA + BMI | 0.822 | (0.748–0.897) | 88.60% | 64.80% | <0.001 |
Logistic ordered multiple regression analysis to find independent risk factors. AUC, the area under the ROC curve; CI, confidence interval; PSA, prostate specific antigen; BMI, body mass index; ROC, receiver operating characteristic.
Discussion
T staging reflects the extent of a tumor in the prostate gland, and plays a key part in determining the prognosis of PCa. In locally advanced PCa treatment, the T stage is an important basis for judging disease severity and choosing the treatment method. Herein, we analyzed retrospectively the relevant clinical data of 177 patients who had a pathologic diagnosis of PCa at the First Affiliated Hospital of the University of Science and Technology of China from 1st January 2015 to 1st May 2020. Through nonlinear regression and single-factor logistic regression analysis, we found that BMI, the PSA level, Gleason score, hypertension, N stage, and M stage were significantly associated with the T stage. Multiple logistic regression analysis showed that BMI and the PSA level were independent risk factors affecting the T stage of PCa. ROC curves also revealed that, although the PSA level was more sensitive than BMI for diagnosing the T stage (71.4% vs. 51.4%), the sensitivity remained low and combination of these two parameters improved the sensitivity significantly (88.6%) but reduced the specificity (64.8%) significantly.
Our study had four important features. First, the inclusion and exclusion criteria were strict, with all participants having pathologically confirmed PCa according to resected specimens. Second, T staging was in strict accordance with guidelines set by the European Association of Urology and International Society of Urological Pathology (in 2014), and Gleason score. Third, all factors affecting the levels of inflammatory biomarkers and the PSA level were excluded. Fourth, the factors that may influence progression of the T-stag were investigated, and locally advanced PCa was studied on the basis of the T stage, which has not been studied in detail previously (or indeed the factors influencing each stage of PCa).
The PSA level is the most commonly used tumor marker for the diagnosis of PCa (14). Some studies have shown a positive correlation between the PSA level and Gleason score (15-18). This correlation may arise from poorly differentiated and highly vicious tissue from patients with a high Gleason score leading to greater damage to the structure of the prostate gland, blood vessels, and lymphatics. This phenomenon would lead finally to more PSA in blood and a higher PSA level in serum. In our study, the PSA level was positively correlated with the T stage. Progression of the T stage has been postulated to increase the tumor size and the extent of destruction of tissue, which may be important factors in the increase in the PSA level. A significant positive correlation between the T stage and Gleason score has been reported (19-21). We suggest that the T stage and Gleason score in locally advanced PCa are important factors in the increase of the PSA level. Therefore, the PSA level can be used to better guide PCa treatment.
The PCa level is closely related to age (22), levels of androgens (23), genetics (24) and obesity (25). Increasing evidence suggests obesity to be associated with an increased incidence of aggressive PCa (9,10,26-30) as well as an increased risk of biochemical failure following radical prostatectomy (31) and external-beam radiotherapy (32), Such results may be associated with a higher prevalence of complications following androgen-deprivation therapy, increased PCa-specific mortality, and difficulties in the treatment of obese men. Obesity may have an impact on the development and progression of PCa by influencing levels of adipokines (33), testosterone (23), and systemic inflammation (34). There is also evidence that PLR as an inflammatory index is a robust prognostic marker in nonmetastatic ccRCC (clear cell renal cell carcinoma) that clearly outperforms other inflammatory indexes in those who had undergone nephrectomy. However, its prognostic effect was limited in the low-risk category of ccRCC (13). While we did not find a significant association between systemic inflammatory indicators (NLR, LMR) and the T stage. Whether this phenomenon is related to dilution due to increased body-fluid volume in obese patients [as suggested by Deng and colleagues (34)] is not known. Using the T stage as an indicator, we noted a significant negative correlation between BMI and T stage progression in locally advanced PCa, which suggested a higher T stage to be associated with lower BMI, which is consistent with the findings of Zhang and co-workers (12). The latter found a higher PSA level and lower BMI among patients suffering from PCa in northwest China, but a mechanism of action was not postulated.
A recent meta-analysis on PSMA-PET (prostate-specific membrane antigen-positron emission tomography) has indicated that compared to traditional imaging exams, PSMA-PET CT (computed tomography) offers greater sensitivity and specificity in tumor staging for patients with intermediate to high-risk PCa; and for screening extra-prostatic invasion and seminal vesicle invasion, PSMA-PET MRI (magnetic resonance imaging) is also more precise and recommended (35). Additionally, compared to PSMA-PET CT, the results of another prospective single-center imaging study suggested that multiparametric MRI is more effective in detecting extra-prostatic extension and seminal vesicle invasion (36). Today, we have a wider range of imaging options to accurately diagnose the staging of PCa in patients. However, a more pressing issue follows: whether patients at their initial diagnosis are willing to bear the high cost of PSMA-PET. Our research, focusing on clinical indicators such as BMI and PSA, which are easier to obtain and less expensive, leads us to believe that in the future, when PSMA-PET becomes more widespread, the integration of these imaging studies with our research could more precisely predict and diagnose the staging of PCa and guide our treatment plans.
Our results suggest that the PSA level is not only important for PCa diagnosis, it may also have an important guiding role in the selection of treatment options and the prognosis. The T stage, combined with BMI and the PSA level, may guide PCa treatment. However, the results of our study are derived from a single center, predominantly comprising individuals of Asian descent from the Eastern region of China. Consequently, there are inherent limitations associated with this study due to the restricted population representation. Additionally, this study is retrospective in nature, leading to potential selection bias, and the BMI included in this research is based on the patients’ BMI during their treatment at the APH. Long-term BMI variations of patients are challenging to obtain. Therefore, to enhance the robustness of our research findings, it is imperative to conduct multi-center, longitudinal investigations over an extended period.
Conclusions
In summary, BMI, and PSA show a significant correlation and benefit in diagnosing and treating locally advanced PCa. They serve as important criteria for assessing severity and prognosis, with higher PSA and lower BMI indicating higher T stage and poorer prognosis. Despite the limitations of our single-center retrospective study, we believe these indicators are vital for assessing locally advanced PCa severity and prognosis. We are dedicated to long-term research to explore and validate their significance. Furthermore, we are actively studying the molecular mechanisms of PCa occurrence, development, and metastasis in relation to adiposity and PSA levels, aligning with existing research on the relationship between PSA, BMI, and PCa. Our research aims to theoretically validate our findings.
Acknowledgments
Funding: The current study was supported in part by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1680/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1680/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1680/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1680/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research protocol was approved (No. 2023-RE-189) by the Ethics Review Committee of the First Affiliated Hospital of the University of Science and Technology of China (Hefei, China). The requirement for patients’ informed consent was waived by the same ethics committee that approved the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Culp MB, Soerjomataram I, Efstathiou JA, et al. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur Urol 2020;77:38-52. [Crossref] [PubMed]
- Bell KJ, Del Mar C, Wright G, et al. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int J Cancer 2015;137:1749-57. [Crossref] [PubMed]
- Haas GP, Delongchamps N, Brawley OW, et al. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol 2008;15:3866-71. [PubMed]
- Matulay JT, DeCastro GJ. Radical Prostatectomy for High-risk Localized or Node-Positive Prostate Cancer: Removing the Primary. Curr Urol Rep 2017;18:53. [Crossref] [PubMed]
- Lee JW, Ryu JH, Kim YB, et al. Do positive surgical margins predict biochemical recurrence in all patients without adjuvant therapy after radical prostatectomy? Korean J Urol 2013;54:510-5. [Crossref] [PubMed]
- Sooriakumaran P, Haendler L, Nyberg T, et al. Biochemical recurrence after robot-assisted radical prostatectomy in a European single-centre cohort with a minimum follow-up time of 5 years. Eur Urol 2012;62:768-74. [Crossref] [PubMed]
- Menon M, Bhandari M, Gupta N, et al. Biochemical recurrence following robot-assisted radical prostatectomy: analysis of 1384 patients with a median 5-year follow-up. Eur Urol 2010;58:838-46. [Crossref] [PubMed]
- Budny A, Grochowski C, Kozłowski P, et al. Obesity as a tumour development triggering factor. Ann Agric Environ Med 2019;26:13-23. [Crossref] [PubMed]
- Tzenios N, Tazanios ME, Chahine M. The impact of body mass index on prostate cancer: An updated systematic review and meta-analysis. Medicine (Baltimore) 2022;101:e30191. [Crossref] [PubMed]
- Saha A, Kolonin MG, DiGiovanni J. Obesity and prostate cancer - microenvironmental roles of adipose tissue. Nat Rev Urol 2023;20:579-96. [Crossref] [PubMed]
- Zorena K, Jachimowicz-Duda O, Ślęzak D, et al. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int J Mol Sci 2020;21:3570. [Crossref] [PubMed]
- Zhang J, Ma M, Nan X, et al. Obesity inversely correlates with prostate-specific antigen levels in a population with normal screening results of prostate cancer in northwestern China. Braz J Med Biol Res 2016;49:e5272. [Crossref] [PubMed]
- Lee A, Lee HJ, Huang HH, et al. Prognostic Significance of Inflammation-associated Blood Cell Markers in Nonmetastatic Clear Cell Renal Cell Carcinoma. Clin Genitourin Cancer 2020;18:304-13. [Crossref] [PubMed]
- Velonas VM, Woo HH, dos Remedios CG, et al. Current status of biomarkers for prostate cancer. Int J Mol Sci 2013;14:11034-60. [Crossref] [PubMed]
- Godtman RA, Kollberg KS, Pihl CG, et al. The Association Between Age, Prostate Cancer Risk, and Higher Gleason Score in a Long-term Screening Program: Results from the Göteborg-1 Prostate Cancer Screening Trial. Eur Urol 2022;82:311-7. [Crossref] [PubMed]
- Palsdottir T, Nordstrom T, Karlsson A, et al. The impact of different prostate-specific antigen (PSA) testing intervals on Gleason score at diagnosis and the risk of experiencing false-positive biopsy recommendations: a population-based cohort study. BMJ Open 2019;9:e027958. [Crossref] [PubMed]
- Nordström T, Akre O, Aly M, et al. Prostate-specific antigen (PSA) density in the diagnostic algorithm of prostate cancer. Prostate Cancer Prostatic Dis 2018;21:57-63. [Crossref] [PubMed]
- Caster JM, Falchook AD, Hendrix LH, et al. Risk of Pathologic Upgrading or Locally Advanced Disease in Early Prostate Cancer Patients Based on Biopsy Gleason Score and PSA: A Population-Based Study of Modern Patients. Int J Radiat Oncol Biol Phys 2015;92:244-51. [Crossref] [PubMed]
- Pan CC, Lee JS, Chan JL, et al. The association between presentation PSA and race in two sequential time periods in prostate cancer patients seen at a university hospital and its community affiliates. Int J Radiat Oncol Biol Phys 2003;57:1292-6. [Crossref] [PubMed]
- Danneman D, Drevin L, Robinson D, et al. Gleason inflation 1998-2011: a registry study of 97,168 men. BJU Int 2015;115:248-55. [Crossref] [PubMed]
- Lehrer S, Diamond EJ, Stagger S, et al. Serum insulin level, disease stage, prostate specific antigen (PSA) and Gleason score in prostate cancer. Br J Cancer 2002;87:726-8. [Crossref] [PubMed]
- Zhang Z, Zhanghuang C, Wang J, et al. Development and Validation of Nomograms to Predict Cancer-Specific Survival and Overall Survival in Elderly Patients With Prostate Cancer: A Population-Based Study. Front Oncol 2022;12:918780. [Crossref] [PubMed]
- van Winden LJ, van Rossum HH. Testosterone analysis in prostate cancer patients. Adv Clin Chem 2022;108:73-104. [Crossref] [PubMed]
- Zarei A, Javid H, Sanjarian S, et al. Metagenomics studies for the diagnosis and treatment of prostate cancer. Prostate 2022;82:289-97. [Crossref] [PubMed]
- Bonn SE, Sjölander A, Tillander A, et al. Body mass index in relation to serum prostate-specific antigen levels and prostate cancer risk. Int J Cancer 2016;139:50-7. [Crossref] [PubMed]
- Purcell SA, Oliveira CLP, Mackenzie M, et al. Body Composition and Prostate Cancer Risk: A Systematic Review of Observational Studies. Adv Nutr 2022;13:1118-30. [Crossref] [PubMed]
- Dickerman BA, Torfadottir JE, Valdimarsdottir UA, et al. Body fat distribution on computed tomography imaging and prostate cancer risk and mortality in the AGES-Reykjavik study. Cancer 2019;125:2877-85. [Crossref] [PubMed]
- Imir OB, Kaminsky AZ, Zuo QY, et al. Per- and Polyfluoroalkyl Substance Exposure Combined with High-Fat Diet Supports Prostate Cancer Progression. Nutrients 2021;13:3902. [Crossref] [PubMed]
- Zhai T. Peri-prostatic adipose tissue measurements using MRI predict prostate cancer aggressiveness in men undergoing radical prostatectomy. J Endocrinol Invest 2021;44:287-96. [Crossref] [PubMed]
- Sato H, Narita S, Ishida M, et al. Specific Gut Microbial Environment in Lard Diet-Induced Prostate Cancer Development and Progression. Int J Mol Sci 2022;23:2214. [Crossref] [PubMed]
- Dahran N, Szewczyk-Bieda M, Vinnicombe S, et al. Periprostatic fat adipokine expression is correlated with prostate cancer aggressiveness in men undergoing radical prostatectomy for clinically localized disease. BJU Int 2019;123:985-94. [Crossref] [PubMed]
- Wong JR, Gao Z, Merrick S, et al. Potential for higher treatment failure in obese patients: correlation of elevated body mass index and increased daily prostate deviations from the radiation beam isocenters in an analysis of 1,465 computed tomographic images. Int J Radiat Oncol Biol Phys 2009;75:49-55. [Crossref] [PubMed]
- Onuma M, Bub JD, Rummel TL, et al. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J Biol Chem 2003;278:42660-7. [Crossref] [PubMed]
- Deng T, Lyon CJ, Bergin S, et al. Obesity, Inflammation, and Cancer. Annu Rev Pathol 2016;11:421-49. [Crossref] [PubMed]
- Chow KM, So WZ, Lee HJ, et al. Head-to-head Comparison of the Diagnostic Accuracy of Prostate-specific Membrane Antigen Positron Emission Tomography and Conventional Imaging Modalities for Initial Staging of Intermediate- to High-risk Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol 2023;84:36-48. [Crossref] [PubMed]
- Sonni I, Felker ER, Lenis AT, et al. Head-to-Head Comparison of (68)Ga-PSMA-11 PET/CT and mpMRI with a Histopathology Gold Standard in the Detection, Intraprostatic Localization, and Determination of Local Extension of Primary Prostate Cancer: Results from a Prospective Single-Center Imaging Trial. J Nucl Med 2022;63:847-54. [Crossref] [PubMed]


