
Bilateral Wilms tumor: 10-year experience from a single center in China
Highlight box
Key findings
• Although nephron sparing surgery (NSS) surgery has higher positive margins, it doesn’t lead to higher tumor recurrence with the preoperative chemotherapy and it should be recommended for bilateral Wilms tumor.
What is known and what is new?
• NSS is recommended for bilateral Wilms tumor.
• Preoperative chemotherapy maybe has a low response rate for bilateral Wilms tumor, but it could improve overall survival. And the customized procedure can be selected according to the location and anatomical features of tumor.
What is the implication, and what should change now?
• After preoperative chemotherapy for bilateral nephroblastoma, nephron-sparing surgery should be performed as much as possible.
Introduction
Wilms tumor is the most common malignant renal tumor in childhood, and about 4% to 8% of cases are bilateral disease with a clinical synchronous or metachronous occurrence (1). Although multidisciplinary collaboration and multimodality therapies have dramatically improved survival for this malignancy (overall survival >85%) (2), the management of bilateral Wilms tumor still faces huge challenges, since bilateral Wilms tumor can have multiple foci and various pathological types: nephroblastoma, nephrogenic rests (NR) and nephroblastomatosis. The latter two types are the precursors of nephroblastoma. The response rate of neoadjuvant chemotherapy varies greatly according to the different pathological types, which affected the choice of subsequent surgery. It is commonly accepted that nephron sparing surgery (NSS) represents a surgical alternative in such patients. It remains a challenge for surgeons to balance the dilemma between complete tumor resection to reduce tumor recurrence and renal parenchyma preservation to reduce end-stage renal disease (ESRD). In this study, we reviewed patients with bilateral Wilms tumors managed in our center and evaluated the influence of different surgical approaches on prognosis and postoperative renal function. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1428/rc).
Methods
Clinical data
The medical records of children with bilateral Wilms tumor who were admitted to our medical center from January 2010 to December 2020 were analyzed retrospectively. The clinical characteristics, imaging data [computed tomography (CT) or magnetic resonance imaging (MRI), radioisotope renogram, ultrasound, etc.], laboratory test results (liver and kidney function, electrolytes, etc.) and surgical conditions (surgical methods, postoperative complications, etc.) were collected. According to the different treatment plans used, the staging system of Children’s Oncology Group (COG) and International Society of Paediatric Oncology (SIOP) were used respectively. Pathology was recorded separately based on the location and number of tumors. The glomerular filtration rate (GFR) was obtained from the results of radioisotope renography, and the normal range is about 80–120 mL/min. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Children’s Hospital of Fudan University [No. 198(2022)] and informed consent was obtained from all legal guardians of the children who agreed to participate in this study.
Treatment algorithm
Open surgical biopsies were performed to provide accurate histological and biological information for patients that were difficult to diagnose solely by imaging. All patients who did not take up-front surgery would receive regimen EE-4A or regimen AV (2019 later) for 2 cycles in 3 weeks as the initial induction therapy. Response after chemotherapy was divided into complete response (tumor volume reduction >75%), partial response (tumor volume reduction between 25% and 75%), stable (tumor volume reduction <25%) and progression (new lesions or tumors volume increased). Radical surgery could be performed as single-stage or two-stage operations according to the tumor location, numbers, and renal function. The approaches of radical resection included radical nephrectomies (RN) and NSS, and the later included partial nephrectomies (PN) and tumor enucleation (TE). If the collecting system was opened, it would be carefully sutured or reconstructed with Double-J catheter inserted. All patients received post-operative chemotherapy according to tumor stage. The COG regimen was used before 2019, and the SIOP regimen was used after 2019. Follow-up was carried out by mail, telephone, online consultation, and clinic. The latest follow-up was December 2021.
Statistical analysis
All the collected data were analyzed using the SPSS, version 18.0. Numerical data were expressed as mean, maximum, and minimum. Qualitative data were expressed as frequency and percentage. Survival curves were presented according to the Kaplan-Meier method. P<0.05 was considered as statistically significant.
Results
Clinical characteristics
From January 2010 to December 2020, a total of 223 patients of Wilms tumor were treated in our hospital, including 16 cases (7.17%) of bilateral lesions. The clinical characteristics of these patients are shown in Table 1. Of the 16 patients, 3 were male and 13 were female. The mean age of patients was 17.88±11.65 months (6–42 months). A palpable abdominal mass was the most common presentation in our study (14 patients), while painless gross hematuria was encountered in the remaining 2 patients. Also, five patients presented with hypertension and two presented with hemihypertrophy. Fifteen cases had synchronous tumors, while only one patient had metachronous bilateral lesions. According to the number of lesions on each kidney, the left and right kidneys with multiple lesions (>1) accounted for 43.8% and 62.5%, respectively. The initial tumor size of the left and right kidney was 532.18±384.25 and 454.84±502.20 mm3, respectively. Among all patients, liver metastasis, inferior vena cava tumor thrombus and tumor rupture occurred in 1 patient each. The creatinine and blood urea nitrogen of all children were normal before treatment, but the GFR of 7 kidneys (left 4, right 3) was found to be less than 20 mL/min. Three patients received whole exome sequencing (WES) and the results showed that there were 1 case of WT1 exon6-7 LOH1 and 2 cases of WT1exon2 heterozygosity in the tumors, respectively.
Table 1
| Clinical characteristics | Values |
|---|---|
| Gender | |
| Male | 3 (18.8) |
| Female | 13 (81.3) |
| Age, months | 17.88±11.65 |
| Concomitant deformity | |
| Hemihypertrophy | 2 (12.5) |
| Hypertension | 5 (31.3) |
| Metachronous patient | 1 (6.3) |
| Glomerular filtration rate of left/right kidney | |
| <20 mL/min | 4 (25.0)/3 (18.8) |
| 20–50 mL/min | 7 (43.7)/8 (50.0) |
| >50 mL/min | 5 (31.3)/5 (31.2) |
| Number of left/right kidney lesions | |
| 1 | 9 (56.2)/6 (37.5) |
| 2 | 1 (6.3)/2 (12.5) |
| 3 | 2 (12.5)/2 (12.5) |
| >3 | 4 (25.0)/6 (37.5) |
| Metastasis | |
| Liver | 1 (6.3) |
| Regional lymph node | 2 (12.5) |
| Inferior vena cava tumor thrombus | 1 (6.3) |
Values are presented as number (percentage) or mean ± standard deviation.
Treatment
Open surgical biopsy was performed in 4 patients, and pathology of the bilateral lesions revealed 3 nephroblastomas, 1 NR, and 4 nephroblastomatosis. Thirteen patients received preoperative chemotherapy and the average number of cycles of preoperative chemotherapy was 3.31±1.89. The results of preoperative chemotherapy varied, consisting of partial response (8 kidneys, 30.8%), stable disease (10 kidneys, 38.4%), and progressive disease (8 kidneys, 30.8%). Only 1 kidney of multiple lesions shrank after preoperative chemotherapy, while the remaining 13 kidneys of multiple lesions (92.9%) remained stable or progresses. After preoperative chemotherapy, 50% (5/10) of the single lesion on the affected kidney was reduced.
Radical tumor resection was performed in the 14 patients, except for 2 patients who gave up treatment due to tumor progression or complications from chemotherapy. Unilateral RN and contralateral biopsy, unilateral RN and contralateral NSS and bilateral NSS were performed in 2, 3 and 9 patients, respectively (Table 2). Single-stage and two-stage operation for bilateral lesions were performed in 7 and 5 patients, respectively. Except for 1 patient with metachronous bilateral lesions, the average interval between two-stage operations in the remaining 4 patents was 3.00±2.83 months (1–2.83 months). For the surgical approaches of the affected kidney, RN, PN and TE were performed in 5, 4 and 17 kidneys respectively. The surgical margins of both RN and PN were negative, and the positive rate of margins after TE was 35.3% (6/17). Unilateral RN were performed in 2, 1 and 2 patients due to low GFR (<20 mL/min), metachronous occurrence and invading invasion the renal hilum, respectively. Ten sides of renal pelvis were repaired during the operation, and Double-J catheter were inserted in all of them.
Table 2
| Treatment and prognosis details | No. of patients or rate |
|---|---|
| Preoperative chemotherapy* | |
| COG Regimen DD-4A | 7 |
| COG Regimen IEV | 2 |
| SIOP Regimen AV | 4 |
| Preoperative biopsy | 4 |
| Surgery | |
| Unilateral RN and contralateral biopsy | 2 |
| Unilateral RN and contralateral NSS | 3 |
| Bilateral NSS | 9 |
| Lymph metastases | |
| Positive | 2 |
| Negative | 12 |
| Postoperative chemotherapy | |
| COG Regimen DD-4A | 5 |
| COG Regimen EE-4A | 2 |
| COG Regimen I | 2 |
| SIOP Regimen AV2 | 5 |
| Postoperative radiotherapy | 2 |
| Recurrence | |
| Positive margins | 1 |
| Negative margins | 1 |
| Survival rate, % | |
| Overall | 78.1 |
| Event-free | 58.6 |
*, only 13 patients received preoperative chemotherapy. COG, Children Oncology Group; SIOP, International Society of Paediatric Oncology; Regimen DD-4A, actinomycin D + vincristine + doxorubicin; Regimen IEV, ifosfamide + etoposide + vincristine; Regimen EE-4A, actinomycin D + vincristine; Regimen AV, actinomycin D + vincristine; Regimen I, cyclophosphamide + etoposide+ vincristine + doxorubicin; Regimen AV2, actinomycin D + vincristine; RN, radical nephrectomy; NSS, nephron sparing surgery.
All patients had bilateral favorable histology, and 8 patients (50%) had different pathological types on both sides. The pathology of bilateral lesions is shown in Table 3. And the subtypes of nephroblastoma include 5 epithelial types, 3 embryonic types, 6 stromal types, and 7 mixed types. Positive lymph nodes involvement was found in 2 patients. Postoperative urine leakage occurred in 8 patients (57.1%), and the average leakage time was 5.13±2.42 days (3–10 days). A total of 8 patients underwent postoperative radioisotope renography. Among them, the unilateral GFR of 2 patients was lower than that of pre-operation, and GFR of the rest kidneys were improved. One patient developed renal insufficiency 12 months after surgery, and the surgical approach was unilateral RN and contralateral NSS simultaneously. All patients received post-operative chemotherapy, including 5 patients with COG regimen DD-4A, 2 patients with COG regimen EE-4A, 2 patients with COG regimen I, and 5 patients with SIOP regimen AV2 (Table 2). Two patients received postoperative radiotherapy due to regional lymph node invasion and pre-operative tumor rupture.
Table 3
| Tumor site | Response to neoadjuvant chemotherapy& | Surgical approaches* | Histology# | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduction | Stable | Progression | RN | PN | TE | NS | Nephroblastoma | NR | Nephroblastomatosis | |||
| Right lesion | ||||||||||||
| Single | 2 | 0 | 1 | 1 | 0 | 4 | 1 | 5 | 1 | 0 | ||
| Multiple | 1 | 5 | 2 | 0 | 0 | 7 | 3 | 4 | 2 | 3 | ||
| Left lesion | ||||||||||||
| Single | 3 | 1 | 3 | 2 | 2 | 5 | 0 | 8 | 1 | 0 | ||
| Multiple | 0 | 4 | 2 | 2 | 2 | 1 | 2 | 4 | 4 | 0 | ||
&, only 13 patients received neoadjuvant chemotherapy; *, 2 patients did not undergo radical surgery; #, the other 2 patients only underwent left radical surgery. RN, radical nephrectomies; PN, partial nephrectomies; TE, tumor enucleation; NS, no surgery; NR, nephrogenic rests.
Follow-up and prognosis
The follow-up time was 6–76 months (mean 26.31±20.39 months). Of the 16 patients, 3 were lost to follow-up and 3 patients were dead. Postoperative recurrence occurred in 2 patients, both of which were unilateral recurrence. There was one recurrence after TE and PN, respectively. Tumor recurrence intervals were 2 and 5 months, respectively, and each for negative and positive margins. Thus, the recurrence rates for negative and positive margins were 12.5% and 16.7%, respectively.
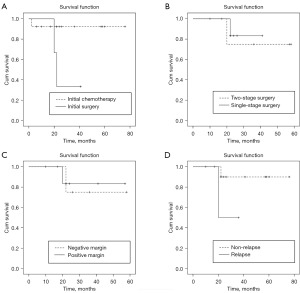
The 5-year overall and event-free survival rates were 78.1% and 58.6%, respectively (Figure 1). The survival rate in the initial chemotherapy group (92.3%) was significantly higher than that in the initial surgery group (33.3%) (P=0.048) (Figure 2A). Positive margin and two-stage operation (P>0.05) appeared no significantly associated with overall survival (Figure 2B,2C). The survival rate of non-relapsed patients was higher than that of relapsed patients (90.0% vs. 50.0%) (Figure 2D) in this study, although there was no statistical difference (P=0.115).
Discussion
Bilateral Wilms tumor is clinically rare, accounting for about 5–8% of Wilms tumor (3,4). Bilateral disease can be synchronous or metachronous, which occurs in 6.3% and 0.85% Wilms tumor patients respectively (3,4). The onset age of bilateral Wilms tumor was earlier than that of unilateral disease (mean 30 vs. 39 months) (3-5).
The results of this study were similar with those reported in the literature. The proportion of bilateral Wilms tumor was 7.17%, and the age of onset (17.88 months) was significantly smaller than the unilateral lesions (38 months) that we reported in the past (6). In this study, 2 patients (12.5%) had deformities associated with hemihypertrophy. Bilateral Wilms tumor is prone to be associated with various anomalies or syndromes, whilst over 100 syndromic associations are described (7). Approximately 5% to 22% patients of bilateral Wilms tumor have urogenital malformation, absence of the iris, WAGR syndrome, Denys-Drash syndrome, hemihypertrophy, or other overgrowth syndromes (8-10). Therefore, for patients who were initially diagnosed with unilateral Wilms tumor, if they were accompanied by related syndromes, it was necessary to pay attention to the possibility of metachronous lesions.
NRs refer to the residual of embryonic renal tissue that remains after 36 weeks of gestation and is considered a precursor lesion to Wilms tumor. Multiple or diffuse NR are defined as nephroblastomatosis, and some nephroblastomatosis could spontaneously regress. In the National Wilms tumor study (NWTS), multiple NR or nephroblastomatosis can be present in approximately 70% of simultaneous bilateral Wilms tumors (11,12), so multiple lesions are more likely to be detected on imaging for bilateral Wilms tumors. In this study, we found that multiple lesions (>1) accounted for 43.8% and 62.5% in the left and right kidneys, respectively. According to the pathological results, it was found that most of the lesions also conformed to the characteristics of NR and nephroblastomatosis. Therefore, the multiple lesions found in bilateral Wilms tumor are more likely to be NR or nephroblastomatosis.
At present, COG believes that imaging examination can identify whether there is bilateral renal lesion, and most of the pathological types are Wilms tumor or NR, while other pathological types are extremely rare. In addition, even if the pathological type is clarified, the treatment plan will not change greatly, but biopsy will change the local stage of the tumor to stage III, so COG does not advocate pre-treatment biopsy including fine needle biopsy (13). In this study, 4 tumor biopsies were performed in the earlier period, and the pathological type of the lesions was also confirmed to be Wilms tumor or NR, and 75% of the patients had different pathological types on both sides, which was consistent with the characteristics of bilateral Wilms tumor. Therefore, we now mainly relied on imaging to diagnose bilateral Wilms tumor.
For the treatment of bilateral Wilms tumor, United Kingdom Children’s Cancer Study Group (UKCCSG) found that initial chemotherapy and initial surgery had similar survival rates, but initial chemotherapy had a lower incidence of renal insufficiency (6%) than initial surgery (20%) (14). In addition, the SIOP 93 study also found that 80 days of preoperative chemotherapy can make 66% of bilateral Wilms tumors eligible for NSS (9). Therefore, for the treatment of bilateral Wilms tumor, COG and SIOP have reached a consensus: radical surgery should be performed after neoadjuvant chemotherapy. There is no unified opinion on the preoperative chemotherapy regimen and course of treatment, but it is generally believed that the three-drug regimen is superior to the two-drug regimen in terms of tumor volume reduction (15). The AREN0543 study of COG found that 84% of bilateral Wilms tumors could undergo surgery within 12 weeks (16). Continuing preoperative chemotherapy after 12 weeks will not only fail to cause further tumor size reduction, but also induce development of anaplasia. Therefore, it is not recommended to prolong chemotherapy beyond 12 weeks. The preoperative chemotherapy in this study was performed average of 3.31±1.89 cycles, which was similar to the results of the COG study. However, our study also found that only 30.8% (8 kidneys) of the lesions shrank after preoperative chemotherapy, and the tumors remained stable or even increased in most cases. After further analysis according to the number of lesions on the affected kidney, we found that the proportion of tumor reduction of multiple lesions (7.1%) after preoperative chemotherapy was no better than that of single lesions (50%), which may be related to the fact that the pathological type of multiple lesions is mainly NR, which was a precursor lesion of Wilms tumor that was not sensitive to chemotherapy. Therefore, when the response to chemotherapy for multiple lesions was not satisfactory, continuing chemotherapy longer or adding drugs may not improve the outcome.
At present, both COG and SIOP advocate NSS as the preferred surgical approach to preserve the renal parenchyma as much as possible and reduce the occurrence of ESRD. NSS is divided into PR and TE according to the specific operation approaches (17,18). The experience of St Jude Children’s Hospital showed that the positive rate of surgical margins after NSS was 31% and comprehensive treatment did not reduce the tumor recurrence rate and improve the overall prognosis (19). In this study, if the tumor was located at one end of the kidney and did not involve the renal hilum, PN would be performed, and the margins were all negative. If the tumor is multiple lesions of the whole kidney or involved the renal pelvis or renal hilum, TE was performed, and the positive rate of tumor margin was higher (35.3%), but the survival rates of patients with negative and positive margins were similar, and the difference was not statistically significant. RN may be considered in patients in which NSS cannot be performed, such as when the tumor invading most of the kidney, or unfavorable histology. In this study, except for 1 patient of metachronous lesions, RN was performed in the other 2 cases because the tumor could not be separated from the renal hilum.
ESRD is more common in bilateral Wilms tumor, with an overall incidence of about 12%, and a higher incidence in cases with predisposition syndromes such as Denys-Drash syndrome (20,21). Currently, loss of renal parenchyma and potentially nephrotoxic chemotherapeutic agents are considered to be the most common cause of ESRD in children with bilateral Wilms tumor (22). Thus, NSS is crucial for reducing the incidence of ESRD in children with bilateral Wilms tumor. A study demonstrated a slight increase in GFR up to the third decade of life in Wilms tumor survivors who underwent NSS compared with RN (23). In this study, postoperative renal insufficiency was found in one patient, who received RN on one side and NSS on the other side simultaneously. In addition, in 6 of the 8 children who underwent postoperative isotope renogram, the GFR were all improved compared with those pre-operation, which reflected that NSS can further protect and improve renal function for bilateral Wilms tumor.
The current prognosis of bilateral Wilms tumor is still unsatisfactory, and the survival rate is much lower than that of unilateral Wilms tumor. In the NWTS-5 study, the 4-year event-free survival rate for bilateral Wilms tumor was 56%, and the 4-year event-free survival rates for favorable histology, focal anaplastic and diffuse anaplastic lesions were 65%, 75% and 25%, respectively (24). With the optimization of the treatment, the AREN0534 study successfully improved outcomes of bilateral Wilms tumor, with 4-year EFS and OS estimates of 82.1% and 94.16% (13). However, the SIOP study also found that although the 5-year overall survival rate can reach 90%, the recurrence-free survival rate and the event-free survival rate are only 38% and 31% (25), so the recurrence and residual of bilateral Wilms tumor were still important factors affecting survival rates. The 5-year survival rate of bilateral Wilms tumor in this study is still unsatisfactory. On the one hand, the proportion of patients lost to follow-up and treatment abandonment is high, and on the other hand, some patients with positive resection margins do not receive radiotherapy, which ultimately affects the overall survival rate and event-free survival rates. However, the survival rate in the initial chemotherapy group (92.3%) was significantly higher than that in the initial surgery group (33.3%), which supported treatment strategies of preoperative chemotherapy. Although the risk of positive margins is high after NSS, the tumor recurrence rate is not very high. Some scholars retrospectively analyzed the SIOP2001 clinical research database and found that the 5-year recurrence rate of bilateral Wilms tumor in the low- and intermediate-risk group was only 13.3%, and that in the high-risk group was 27.1% (26). The 2 patients of recurrence in this study all received NSS, and the recurrence rates of negative and positive margins were similar. These indicate that even if there are residual or positive margins during NSS operation, it will not significantly increase the recurrence rate, which may be related to the lack of viable tumor cells after treatment of residual disease.
Several limitations of this study deserve mention. This is a retrospective single-institution study with a small number of cases and a limited period of follow-up. Moreover, the proportion of patients lost to follow-up is relatively high, which will affect the statistical results of survival rate. In addition, most patients with positive resection margins do not receive radiotherapy, which will also affect the survival rate of these patients.
Conclusions
The proportion of tumor reduction after preoperative chemotherapy is relatively low. Preoperative chemotherapy has a low response rate for bilateral Wilms tumor, but preoperative chemotherapy could improve overall survival. NSS is recommended for bilateral Wilms tumor, and the customized procedure can be selected according to the location and anatomical features of tumor.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1428/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1428/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1428/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1428/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Children’s Hospital of Fudan University [No. 198(2022)] and informed consent was obtained from all legal guardians of the children who agreed to participate in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dome JS, Graf N, Geller JI, et al. Advances in Wilms Tumor Treatment and Biology: Progress Through International Collaboration. J Clin Oncol 2015;33:2999-3007. [Crossref] [PubMed]
- Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014;64:83-103. [Crossref] [PubMed]
- Breslow N, Olshan A, Beckwith JB, et al. Epidemiology of Wilms tumor. Med Pediatr Oncol 1993;21:172-81. [Crossref] [PubMed]
- Neville HL, Ritchey ML. Wilms' tumor. Overview of National Wilms' Tumor Study Group results. Urol Clin North Am 2000;27:435-42. [Crossref] [PubMed]
- Ritchey ML, Shamberger RC, Hamilton T, et al. Fate of bilateral renal lesions missed on preoperative imaging: a report from the National Wilms Tumor Study Group. J Urol 2005;174:1519-21; discussion 1521. [Crossref] [PubMed]
- Yao W, Li K, Xiao X, et al. Outcomes of Wilms' tumor in eastern China: 10 years of experience at a single center. J Invest Surg 2012;25:181-5. [Crossref] [PubMed]
- Charlton J, Irtan S, Bergeron C, et al. Bilateral Wilms tumour: a review of clinical and molecular features. Expert Rev Mol Med 2017;19:e8. [Crossref] [PubMed]
- Indolfi P, Jenkner A, Terenziani M, et al. Synchronous bilateral Wilms tumor: a report from the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP). Cancer 2013;119:1586-92. [Crossref] [PubMed]
- Sudour H, Audry G, Schleimacher G, et al. Bilateral Wilms tumors (WT) treated with the SIOP 93 protocol in France: epidemiological survey and patient outcome. Pediatr Blood Cancer 2012;59:57-61. [Crossref] [PubMed]
- Kim JK, Hansen A, Peard L, et al. Bilateral Wilms Tumor in CLOVES Syndrome. Urology 2023;177:178-80. [Crossref] [PubMed]
- Welter N, Brzezinski J, Treece A, et al. The pathophysiology of bilateral and multifocal Wilms tumors: What we can learn from the study of predisposition syndromes. Pediatr Blood Cancer 2023;70:e29984. [Crossref] [PubMed]
- Coppes MJ, Arnold M, Beckwith JB, et al. Factors affecting the risk of contralateral Wilms tumor development: a report from the National Wilms Tumor Study Group. Cancer 1999;85:1616-25. [Crossref] [PubMed]
- Ehrlich P, Chi YY, Chintagumpala MM, et al. Results of the First Prospective Multi-institutional Treatment Study in Children With Bilateral Wilms Tumor (AREN0534): A Report From the Children's Oncology Group. Ann Surg 2017;266:470-8. [Crossref] [PubMed]
- Kumar R, Fitzgerald R, Breatnach F. Conservative surgical management of bilateral Wilms tumor: results of the United Kingdom Children's Cancer Study Group. J Urol 1998;160:1450-3. [Crossref] [PubMed]
- Dome JS, Perlman EJ, Graf N. Risk stratification for wilms tumor: current approach and future directions. Am Soc Clin Oncol Educ Book 2014;215-23. [Crossref] [PubMed]
- Godzinski J, Graf N, Audry G. Current concepts in surgery for Wilms tumor--the risk and function-adapted strategy. Eur J Pediatr Surg 2014;24:457-60. [Crossref] [PubMed]
- Kieran K, Davidoff AM. Nephron-sparing surgery for bilateral Wilms tumor. Pediatr Surg Int 2015;31:229-36. [Crossref] [PubMed]
- Davidoff AM, Giel DW, Jones DP, et al. The feasibility and outcome of nephron-sparing surgery for children with bilateral Wilms tumor. The St Jude Children's Research Hospital experience: 1999-2006. Cancer 2008;112:2060-70. [Crossref] [PubMed]
- Breslow NE, Collins AJ, Ritchey ML, et al. End stage renal disease in patients with Wilms tumor: results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol 2005;174:1972-5. [Crossref] [PubMed]
- Aldrink JH, Heaton TE, Dasgupta R, et al. Update on Wilms tumor. J Pediatr Surg 2019;54:390-7. [Crossref] [PubMed]
- Hamilton TE, Ritchey ML, Haase GM, et al. The management of synchronous bilateral Wilms tumor: a report from the National Wilms Tumor Study Group. Ann Surg 2011;253:1004-10. [Crossref] [PubMed]
- Cozzi DA, Ceccanti S, Frediani S, et al. Renal function adaptation up to the fifth decade after treatment of children with unilateral renal tumor: a cross-sectional and longitudinal study. Pediatr Blood Cancer 2013;60:1534-8. [Crossref] [PubMed]
- Dome JS, Cotton CA, Perlman EJ, et al. Treatment of anaplastic histology Wilms' tumor: results from the fifth National Wilms' Tumor Study. J Clin Oncol 2006;24:2352-8. [Crossref] [PubMed]
- Grundy PE, Breslow NE, Li S, et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol 2005;23:7312-21. [Crossref] [PubMed]
- Mavinkurve-Groothuis AM, van den Heuvel-Eibrink MM, Tytgat GA, et al. Treatment of relapsed Wilms tumour (WT) patients: experience with topotecan. A report from the SIOP Renal Tumour Study Group (RTSG). Pediatr Blood Cancer 2015;62:598-602. [Crossref] [PubMed]
- Brok J, Lopez-Yurda M, Tinteren HV, et al. Relapse of Wilms' tumour and detection methods: a retrospective analysis of the 2001 Renal Tumour Study Group-International Society of Paediatric Oncology Wilms' tumour protocol database. Lancet Oncol 2018;19:1072-81. [Crossref] [PubMed]



