
Chimeric antigen receptor T cells get passed by leukemia
New immunotherapy treatments have led to dramatic responses in patients with chemotherapy-refractory solid tumors, leukemias, and lymphomas. One of these immunotherapies involves the adoptive transfer of autologous T cells gene-engineered to express a chimeric antigen receptor (CAR) that is a hybrid of an antibody and a T cell receptor (TCR) (1). The antibody portion provides new antigen specificity and after binding, the intracellular TCR domains of the CAR induce T cell activation and tumor killing. CAR T cells targeted against CD19, a protein expressed on nearly all normal and cancerous B cells, has mediated impressive responses in patients with relapsed/refractory B cell acute lymphoblastic leukemia (B-ALL). Four groups have reported complete remission (CR) rates up to 90% in pediatric and/or adult patients with B-ALL, while the expected CR rate with chemotherapy alone is <30% (2-5). The number of patients treated with anti-CD19 CAR T cells has increased to beyond 100 and novel forms of relapse that allow tumor cells to avoid killing by CAR T cells have been identified. Although anti-CD19 CAR T cell therapy remains at an early stage of development, an understanding of the mechanisms of treatment escape may identify patients at high-risk for relapse and uncover new avenues to circumvent these relapses.
Gardner et al. (6) recently reported details from two relapses out of seven patients with MLL B-ALL treated with anti-CD19 CAR T cell therapy. Both cases were characterized as having ALL to AML lineage switch shortly after CAR T cell therapy indicating the role of phenotype switch in therapy failure. In one case there was retention of the IGH rearrangement after relapse and lineage switch suggesting de-differentiation of a B lymphoid clone. In the other case the IGH rearrangement was not detected in relapsed AML blasts, suggesting a non-committed precursor or selection of a preexisting myeloid clone. The MLL chromosomal translocation involving chromosome 11, band q23 (7) confers a poor prognosis in chemotherapy treated patients and is known to associate with lymphoid to myeloid lineage switch, often shortly after chemotherapy (8). In contrast, out of 62 cases of non-MLL rearranged B-ALL after anti-CD19 CAR T cell therapy none exhibited lineage switch.
The mechanism for lineage conversion of MLL B-ALL after therapy is unclear and possibilities include: early non-committed clonal hematopoietic progenitors able to give rise to either lineage; lymphoid stem cell clone plasticity allowing lineage inter-conversion; or the presence of a bi-lineal population of blasts present at diagnosis, which upon therapeutic selection, leads to emergence of a minor alternate subset (9). These putative mechanisms allow hypotheses to be developed and tested to determine if any may be involved after CD19-targeted CAR T cell treatment (Figure 1). This may require more extensive immunophenotypic and genetic characterizations of the leukemia immediately before and after CAR T cell treatment. While these analyses will take time, the report from Gardner et al. (6) must give any CAR T cell clinician pause prior to the use of anti-CD19 CAR T cell therapy for patients with a MLL-rearranged B-ALL. The quick rate of relapse suggests that clinical trials should evaluate the utility of allogeneic stem cell transplants immediately after anti-CD19 CAR T cell therapy for MLL B-ALL. Using conventional chemotherapy, such an approach can act as a bridge for relapsed and refractory leukemia patients not expected to achieve remissions with chemotherapy alone (10).
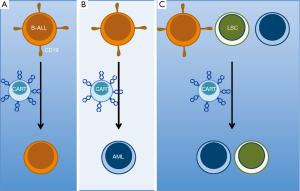
Lineage-switch is just one mechanism of immune escape after CD19-targeted CAR T cell therapy. Relapses after blinatumomab, a CD19/CD3 bi-specific T cell engager recently FDA approved for B-ALL, harbor CD19-negative clones in 50% of cases (11). This loss of epitope (i.e., antigen) is now being detected after treatment with CD19-targeted CAR T cells as well (3). Recent work published by Sotillo et al. (12) elucidated one origination for epitope/antigen loss via an alternative splice mechanism. They demonstrated that in several cases relinquishment of the CAR targeted CD19 surface epitope was due to the emergence of variant CD19 epitope. This N-terminally truncated variant is encoded by mRNA having missense mutations within, or complete loss of, exon 2. The resulting leukemic clones no longer express the CD19 CAR T cells cognate epitope, yet the alternate CD19 isoform at least partially rescues defects in cell signaling and proliferation associated with complete loss of CD19. These CD19 variants are associated with a decrease in SRSF3, a splicing factor they elegantly demonstrated is essential for splicing exon 2 of CD19. This mechanism of resistance to targeted therapy was similarly demonstrated with aberrantly spliced BRAF (V600E) mediating resistance to vemurafenib (13). In the future new therapies should be developed to combat this mechanism of CD19 epitope loss. One option could include CARs targeting CD19 extracellular domains other than exon 2.
These recent reports identify novel forms of ALL resistance to CD19-targeted CAR T cell therapy. Considering recent successes with CD19-targeted therapies for B-ALL, diffuse large B cell lymphoma, and mantle cell lymphoma (14,15) it is likely this therapy will be approved for disease control in patients with B cell malignancies. Therefore, we foresee a significant need to anticipate and potentially prevent relapses in these patients to preserve overall survival. To avoid immune escape by antigen-downregulation or epitope loss: bi-specific targeting with two CARs against distinct epitopes on the same antigen or even different antigens altogether could be employed. In the case of myeloid-switch relapses the potential interventions will depend on the mechanism of relapse. If there are de novo heterogenous myeloid and lymphoid blasts at the time of treatment then targeting both with separate CD19-specific and myeloid-specific CARs would be indicated. In contrast, if the mechanism of relapse is de-differentiation then serial treatments first with a CD19-specific CAR followed by a myeloid-specific CAR would be indicated. Considering that trials testing such theories may not be available for some time, we strongly suggest that for the near-term any patient with a MLL-rearranged B-ALL should be considered for allogeneic hematopoietic stem cell transplant after treatment with anti-CD19 CAR T cells even if in CR. The report by Gardner et al. (6) revealed that utilizing anti-CD19 CARs to treat MLL B-ALL may not get the patient all the way to a durable CR. This report also allows us to speculate on new ways to make this treatment more successful for MLL B-ALL patients: taking CARs on a bridge to allogeneic transplant or selecting a new ride on CARs targeting myeloid blasts toward prolonged remissions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xia Fang (Department of Hematology, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: Dr. Locke has attended Advisory Board Meetings for Kite Pharma. Dr. Davila is a DSMB member for various CAR T cell clinical trials.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013;3:388-98. [Crossref] [PubMed]
- Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25 [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517-28. [Crossref] [PubMed]
- Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123-38. [Crossref] [PubMed]
- Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016;127:2406-10. [Crossref] [PubMed]
- Ziemin-van der Poel S, McCabe NR, Gill HJ, et al. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci U S A 1991;88:10735-9. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press, 2008.
- Rossi JG, Bernasconi AR, Alonso CN, et al. Lineage switch in childhood acute leukemia: an unusual event with poor outcome. Am J Hematol 2012;87:890-7. [Crossref] [PubMed]
- Locke F, Agarwal R, Kunnavakkam R, et al. A novel clofarabine bridge strategy facilitates allogeneic transplantation in patients with relapsed/refractory leukemia and high-risk myelodysplastic syndromes. Bone Marrow Transplant 2013;48:1437-43. [Crossref] [PubMed]
- Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011;29:2493-8. [Crossref] [PubMed]
- Sotillo E, Barrett DM, Black KL, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov 2015;5:1282-95. [Crossref] [PubMed]
- Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 2011;480:387-90. [Crossref] [PubMed]
- Locke FL, Neelapu SS, Bartlett NL, et al. Updated Phase 1 Results From ZUMA-1: A Phase 1-2 Multicenter Study Evaluating the Safety and Efficacy of KTE-C19 (Anti-CD19 CAR T Cells) in Subjects With Refractory Aggressive Non-Hodgkin Lymphoma. Molecular Therapy 2016;24:S294.
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540-9. [Crossref] [PubMed]


