Impact of metastasis site for survival of patients with advanced thymic epithelial tumors
Introduction
Thymic epithelial tumors (TET), including thymoma and thymic carcinoma, are the most common anterior mediastinal neoplasm (1). The clinical behavior of TET has been reported to vary from benign to malignance (2). Surgery is the standard treatment for TET, and complete resection is the first option for patients without distant metastasis (3). For patients with metastasis, the survival was poor and overall survival ranged from 10 to 40 months (4).
For patients of TET with recurrence or advanced stage, knowledge regarding prognostic factors was limited due to its rarity. Most of published experience with prognostic data was based on case reports or studies with limited number participants (5-8). It is well known that tumor stage is an extremely important factor which influences the survival in TET (9). Patients with advanced stage are an unfavorable factor that decreased the survival time. According to the tumor stage, there are different metastasis sites in advanced disease. There were diverse survival results among different distant metastasis sites in many solid carcinomas (10). However, no study focused on the impact of metastasis sites for survival of advanced TET.
In present study, we reviewed a series of consecutive patients with advanced TET who had been treated in our hospital to evaluate impact of the metastasis site to the overall survival.
Methods
Patient eligibility
Patients with pathologic stage IV TET, who diagnosed between January 2005 and July 2015, were retrospectively documented in Zhejiang Cancer Institution. The histologic types were based on the 2004 WHO classification and three pathologists reviewed all of the samples. Patients’ clinical stages determined according to the Masaoka-Koga staging system (11). The metastasis sites were confirmed using chest/abdomen CT and other routine examinations. The present study was approved by ethics committee of Zhejiang Cancer Hospital.
Clinical efficacy evaluation
Response to chemotherapy was assessed by RECIST criteria version 1.1. Objective tumor responses included complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). The disease control rate (DCR) was defined as the sum of the objective responses and stabilization rates (CR + PR + SD).
Statistical analysis
Survival was evaluated from the first day of confirming the advanced stage to the date of death or that of the last follow-up visit. Progression-free survival (PFS) encompassed the time from the first day of chemotherapy to the time of progression or death. Survival curves were calculated using the Kaplan-Meier method and comparison with log-rank. Multivariate analysis was performed using the Cox regression model. Statistical analysis was performed with the SPSS 18 software (Inc., Chicago, IL, USA). Values of P<0.05 were considered significant. The last follow-up time was Mar 1, 2016. The median follow-up period was 49.5 (10.5–105.0) months.
Results
Patient characteristics
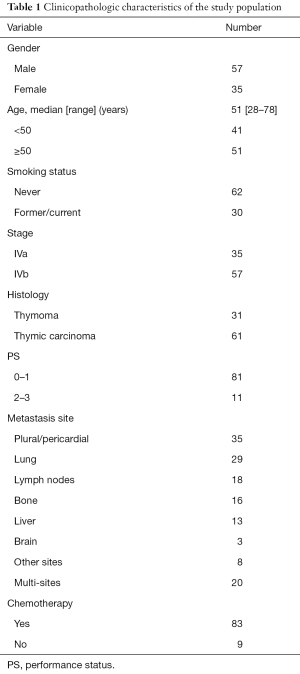
Ninety two patients were recruited in present study. There were 57 males and 35 females, with a median age of 51 years at advanced stage diagnosis (range, 28–78 years). Thirty-five patients were with stage IVa and 57 with IVb. Histologic examination revealed 31 patients had thymoma and 61 with thymic carcinoma. For thymoma patients, there were 1 with type A (1.1%), 1 with type AB (1.1%), 2 with type B1 (2.2%), 11 with type B2 (12.0%), 16 with type B3 thymoma (17.4%) and 61 with thymic carcinoma (66.3%). Among the 61 patients with thymic carcinoma, histologic examination demonstrated the following subtypes: 37 patients (60.7%) had squamous cell carcinoma, 8 (13.1%) had undifferentiated carcinoma, 6 (9.8%) had neuroendocrine carcinoma, 2 (3.3%) had small cell carcinoma, and 8 (13.1%) had carcinoma of other types. Eastern Cooperative Oncology Group performance status (PS) 0–1 was present in 81 patients and PS 2–3 accounted for 11 patients. The most common site of metastasis was plural/pericardial (n=35), followed by lung (n=29), lymph nodes (n=18), bone (n=16), liver (n=13), brain (n=3) and other sites (n=8). Among these, 20 were with multi-sites metastasis. The clinical characteristics of present study are listed in Table 1.
Full table
Treatment and clinical efficacy
Among the 92 patients, 53 had surgery history, 39 were with advanced stage at first presentation. Eighty-three received chemotherapy in first-line treatment, including 72 with doublet regimens or multiagent and 11 were with single agent. Nine patients were with supportive treatment for poor PS or refused treatment. Forty-five patients had received second-line or further-line chemotherapy after failure of prior treatment. Thirty-five patients received radiotherapy during the treatment period.
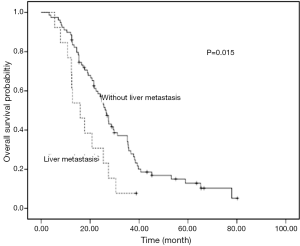
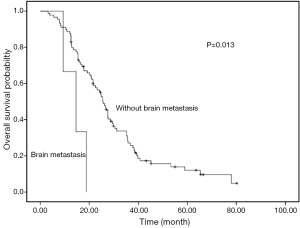
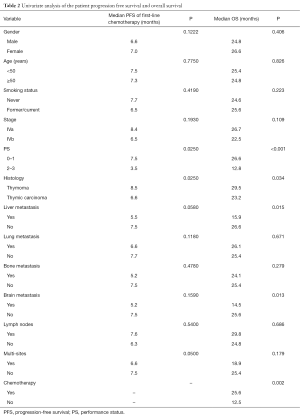
For the 83 patients received first-line chemotherapy, the median PFS was 7.5 months (95% CI: 6.5–8.5). The response rate and DCR were 48.2% and 81.9%, respectively. There was a trend of PFS difference between patients with and without liver metastasis (5.5 vs. 7.5 months, P=0.058). A same trend was found in patients with and without brain metastasis (5.2 vs. 7.5 months, P=0.159). But, no PFS difference was documented in other metastasis sites (Table 2).
Full table
Survival analyses
Univariate analyses were used to evaluate the predictive capability of each variable influencing OS. Gender, age, and smoking were not found to be statistically associated with OS (Table 2). Histology (P=0.034), liver metastasis (P=0.015), brain metastasis (P=0.013), chemotherapy (P=0.002) and PS (P<0.001) were predictive factor of OS (Figures 1,2). Other factors, including age, gender, smoking and stage were not associated with OS. The survival time in patients with different metastasis sites was listed in Table 2.
Among 61 patients with thymic carcinoma, two patients were with brain metastasis. A trend of OS difference existed in patients with and without brain metastasis (14.5 vs. 24.6 months, P=0.187). Seven patients of thymic carcinoma were with liver metastasis. Similarly, a trend of shorter OS was observed in patients with liver metastasis (12.4 vs. 24.8 months, P=0.118).
Among 31 patients with thymoma, one patient (type B2) was with brain metastasis. A shorter OS was observed in patients with brain metastasis (9.3 vs. 29.5 months, P<0.001). Six patients of thymoma were with liver metastasis. A significant OS difference was found in patients with and without liver metastasis (15.9 vs. 36.7 months, P=0.008). The types of thymoma in patients with liver metastasis were as follows: type B2 (n=2), type B3 (n=2), type B1 (n=1) and type AB (n=1).
A multivariate Cox regression model was constructed with the incorporation of PS, histology, liver metastasis, brain metastasis, chemotherapy to evaluate the OS. PS (P=0.001), brain metastasis(P=0.008), histology (P=0.011) and liver metastasis (P=0.015) remained as independent prognostic factors (Table 3).
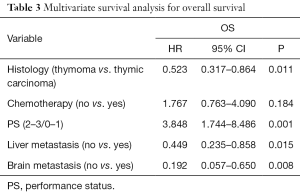
Full table
Discussion
Our results suggest that liver and brain metastasis were unfavorable factors for overall survival of advanced stage TET patients. To our knowledge, this is the first study to evaluate the metastasis site as prognostic factors in advanced TET.
For patients without distant metastasis, surgery is usually the first option for TET patients. Although with a favorable prognosis for patients after surgery, few patients may recur or metastasis in TET patients. The recurrence rate of thymic carcinoma was ranged from 25.4% to 50.4% in our previous studies according to different stage (12,13). Most of patients with advanced or recurrence were with histology of thymic carcinoma, however, patients with thymoma could be recurrence even after compete resection. In our previous study, we found 37.2% patients were with recurrence after surgery in patients with type B2 thymoma (14). For patients with recurrence or metastasis, palliative chemotherapy or radiotherapy may be a better option, which was identified to be prolonged the survival time for these patients (15-17). Although many regimens are used in first-line chemotherapy, no standard regimen was recommended as high level due to lack randomized controlled study with large number patients (4). Both of anthracycline-based and platinum-based treatments are widely used in routine practice (4). In current study, we found chemotherapy could be prolonged the survival time compared with supportive care treatment. However, no efficacy difference was found between different regimens.
Few studies were conducted to identify the prognostic factors in advanced TET due to its rarity. Histology was a significant prognostic factor for survival of TET patients in several previous studies (18). However, there was some controversial in another study (19). In present study, significant difference was observed between thymoma and thymic carcinoma. For the impact of metastasis site for survival of TET patients, most of previous studies were based on case reports, cases series focused on metastasis sites were not widely found (20,21). A retrospective study by Okuma et al. (19) demonstrated that there was a trend of longer time survivals in patients with lung metastasis, but, details difference was not reported. In current study, we found lung metastasis was not a worse factor for survivals of TET compared with other metastasis sites. Liver metastasis was an unfavorable prognostic factor in many solid tumors. However, no previous studies have reported the impact of liver metastasis for prognosis of TET. Our results showed that liver metastasis was a poor prognostic factor in advanced TET, which is consistent with other solid carcinomas (22). Brain metastasis was another factor that decreased the survival of patients in many tumors, one of the reason may due to the blood brain barrier that influenced the efficacy of drugs. Only few cases of TET with brain metastasis have been reported in previous studies and the prognosis was unclear (23,24). In current study, three patients were with brain metastasis which accounted for 3.3% of all patients, and the survival time was the shortest compared with patients with other metastasis sites.
The major limitation of current study is its retrospective nature and relatively small sample number patients. Secondly, the treatment regimens were not unified, which may influence the analysis of survival. Thirdly, patients with liver and brain metastasis were limited, which may result the bias of our conclusions. However, due to the rarity of this tumor, our study provides relevant insight into the clinical work.
The results of this study suggest that both of liver and brain metastasis were independent unfavorable prognostic factors for survival of advanced TET patients. These two factors should be considered in clinical practice before systematic treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.35). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The present study was approved by ethics committee of Zhejiang Cancer Hospital (No. IRB-2015-049) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. [Crossref] [PubMed]
- Ströbel P, Hohenberger P, Marx A. Thymoma and thymic carcinoma: molecular pathology and targeted therapy. J Thorac Oncol 2010;5:S286-90. [Crossref] [PubMed]
- Fu H, Gu ZT, Fang WT, et al. Long-Term Survival After Surgical Treatment of Thymic Carcinoma: A Retrospective Analysis from the Chinese Alliance for Research of Thymoma Database. Ann Surg Oncol 2016;23:619-25. [Crossref] [PubMed]
- Wei ML, Kang D, Gu L, et al. Chemotherapy for thymic carcinoma and advanced thymoma in adults. Cochrane Database Syst Rev 2013;CD008588 [PubMed]
- Song Z, Yu X, Zhang Y. Chemotherapy and prognosis in advanced thymic carcinoma patients. Clinics (Sao Paulo) 2015;70:775-80. [Crossref] [PubMed]
- Wu JX, Chen HQ, Shao LD, et al. Long-term follow-up and prognostic factors for advanced thymic carcinoma. Medicine (Baltimore) 2014;93:e324 [Crossref] [PubMed]
- Modh A, Rimner A, Allen PK, et al. Treatment Modalities and Outcomes in Patients With Advanced Invasive Thymoma or Thymic Carcinoma: A Retrospective Multicenter Study. Am J Clin Oncol 2016;39:120-5. [Crossref] [PubMed]
- Gubens MA. Treatment updates in advanced thymoma and thymic carcinoma. Curr Treat Options Oncol 2012;13:527-34. [Crossref] [PubMed]
- Liang G, Gu Z, Li Y, et al. Comparison of the Masaoka-Koga staging and the International Association for the Study of Lung Cancer/the International Thymic Malignancies Interest Group proposal for the TNM staging systems based on the Chinese Alliance for Research in Thymomas retrospective database. J Thorac Dis 2016;8:727-37. [Crossref] [PubMed]
- Amri R, Bordeianou LG, Sylla P, et al. Variations in Metastasis Site by Primary Location in Colon Cancer. J Gastrointest Surg 2015;19:1522-7. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Song Z, Zhang Y. Outcomes after surgical resection of thymic carcinoma: A study from a single tertiary referral centre. Eur J Surg Oncol 2014;40:1523-7. [Crossref] [PubMed]
- Song Z, Zhang Y. Adjuvant therapy in stage II thymic carcinoma. J Cancer Res Clin Oncol 2014;140:349-52. [Crossref] [PubMed]
- Song Z, Jin X, Zhang Y. Treatment and prognosis of type B2 thymoma. World J Surg Oncol 2014;12:291. [Crossref] [PubMed]
- Schmitt J, Loehrer PJ Sr. The role of chemotherapy in advanced thymoma. J Thorac Oncol 2010;5:S357-60. [Crossref] [PubMed]
- Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060-5. [Crossref] [PubMed]
- Song Z, Yu X, He C, et al. Docetaxel-based chemotherapy as second-line regimen for advanced thymic carcinoma. Thorac Cancer 2014;5:169-73. [Crossref] [PubMed]
- Hosaka Y, Tsuchida M, Toyabe S, et al. Masaoka stage and histologic grade predict prognosis in patients with thymic carcinoma. Ann Thorac Surg 2010;89:912-7. [Crossref] [PubMed]
- Okuma Y, Hosomi Y, Takagi Y, et al. Clinical outcomes with chemotherapy for advanced thymic carcinoma. Lung Cancer 2013;80:75-80. [Crossref] [PubMed]
- Kashima J, Horio H, Okuma Y, et al. Osseous oligometastases from thymic carcinoma: a case report suggesting the effectiveness of palliative-intent radiotherapy treatment. Onco Targets Ther 2016;9:1029-32. [PubMed]
- Vernon J, Schieman C, Schneider L, et al. Rare case of subcarinal thymic carcinoma in the middle mediastinum. J Surg Case Rep 2015;2015. pii: rjv030.
- Hayashi M, Inoue Y, Komeda K, et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg 2010;10:27. [Crossref] [PubMed]
- Haryu S, Saito A, Inoue M, et al. Brain metastasis from invasive thymoma mimicking intracerebral hemorrhage: case report. Neurol Med Chir (Tokyo) 2014;54:673-6. [Crossref] [PubMed]
- Ohata N, Usami N, Kawaguchi K, et al. Type AB thymoma with brain metastasis: Report of a case. Surg Today 2011;41:1436-8. [Crossref] [PubMed]