
Roles of squamous cell cancer antigen, cytokeratin 21-1 fragment, and carcinoembryonic antigen in the diagnosis and prognosis of postoperative locoregional recurrence in esophageal carcinoma
Introduction
Esophageal cancer (EC), a disease in which malignant cells form in the esophageal tissues, remains the predominant cancer in east Asia (1,2). Nowadays, radical resection has been considered as the major treatment regimen for EC (3,4). The outcome of patients is not satisfactory with a 5-year survival rate of less than 30.0% due to locoregional recurrence (LR) (5). To our best knowledge, LR is the major cause for poor response after radical resection (6). Therefore, early diagnosis of LR and positive treatment is crucial for the extension of overall survival in EC patients.
The follow up of patient with EC after surgery is mainly depending on the computed tomography (CT) or PET/CT. Unfortunately, these techniques have disadvantages such as invasive, expensive in cost and not suitable for repetitive tests in a short-term (7). To our knowledge, extensive studies have been carried out to investigate the feasibility of serum tumor markers, such as carcinoembryonic antigen (CEA), squamous cell cancer antigen (SCC-Ag), and cytokeratin 21-1 fragment (CYFRA21-1), in the diagnosis, prognosis, or clinical management of malignant diseases (8-10). For example, SCC-Ag has been used for the diagnosis of squamous cell carcinoma, such as nasopharyngeal squamous cell carcinoma, lung squamous cell carcinoma, and squamous carcinoma of the cervix. For CYFRA21-1, it has been commonly used for the screening of non-small cell lung cancer and colon carcinoma. Besides, CEA has been frequently utilized in the diagnosis and treatment evaluation of lung cancer, breast cancer and gastrointestinal tract cancer, respectively. These studies contributed to the diagnosis and treatment evaluation in the initial management of EC, however, the feasibility of these markers in the evaluation of postoperative recurrence and the prognosis of EC patients is still not well defined. In this retrospective study, 62 EC patients with LR after surgery admitted to our hospital were included. We aim to investigate the feasibility of SCC-Ag, CEA and CYFRA21-1 in the diagnosis of postoperative LR and prognosis of EC patients.
Methods
Patients
Sixty-two EC patients with postoperative LR admitted to our department between January 2008 and December 2014 were enrolled in this study. The criteria of exclusion were as follows: (I) those received non-radical resection; (II) concurrent with other types of tumor; (III) received radiotherapy and/or chemotherapy after surgery or until local recurrence; (IV) died from treatment-related complications; (V) those with a Karnofsky Performance Status (KPS) score of less than 60 showed no tolerance to radiotherapy or chemoradiotherapy. Thirty matched healthy individuals received physical examination served as the normal control. Written informed consent was obtained from each patient. The study protocols were approved by the Ethic Committee of Changzhou No. 2 People’s Hospital (Approval No.: 2015 RES 011-01). The participants of the present study did not write informed consent before taking part since this was a retrospective study.
Chemical analysis
Postoperative LR was confirmed using histopathological examination, CT and/or PET/CT. The concentrations of serum tumor markers including SCC-Ag, CYFRA21-1 and CEA were determined before and one month after treatment. SCC-Ag level was evaluated using chemiluminescent microparticle immunoassay with the commercial kit purchased from Abbott Laboratories (CA, USA). CYFRA21-1 was determined using the chemiluminescence assay with the kit purchased from Roche (MA, USA). CEA was determined using the direct chemiluminescence assay with the kit purchased from Siemens (Munich, Germany). All the procedures were carried out with strict adhesion to the manufacturer’s instructions.
Treatment plan
Eight patients (12.9%) received radiotherapy, and the other 54 patients (87.1%) received chemoradiotherapy. For the patients received radiotherapy, the lesions at the deep of the thoracic and abdominal cavity were exposed to three-dimensional conformal radiation therapy or intensity modulated radiation therapy using 6 MV X-ray beams. The cervical and supraclavicular lesions were treated by the mixed irradiation by 6 MV X-ray beams and 9 Mev electron rays with a dosage of 1.8–2.0 Gy once (total dosage: 50–74 Gy). For the chemotherapy, patients received 1–6 cycles of platinum-based chemotherapy.
Follow up and survival analysis
The patients were followed up once every 3–6 months within 3 years after the treatment. The patients were followed up once per year until December 2014 or death. The survival was defined as the duration from presentation of local recurrence to death. Besides, the survival rates were compared in the patients with negative results in the tumor marker screening and those with positivity. The normal ranges for SCC-Ag, CYFRA21-1 and CEA were defined as less than 1.5, 4 and 4.3 ng/mL, respectively.
Statistical analysis
SPSS 16.0 software was used for the statistical analysis. The measurement data were evaluated using Student’s t-test. Sample rates were compared using Chi square test. The survival rates were measured using Kaplan-Meier method, and was tested using Logrank analysis. Multiple logic regression analysis was used for the analysis of prognostic factors. P<0.05 demonstrated significant difference.
Results
Patient characteristics
Sixty-two EC patients with postoperative LR were included in this study (Table 1). The patients (male: 34, female: 28) were aged between 41 and 80 years (median: 64 years). Among these patients, 60 were diagnosed with squamous carcinoma, while 2 were diagnosed with adenocarcinoma. For the postoperative pathological staging, the number of patients of stage I, II and III was 13, 26, and 23, according to the American Joint Committee on Cancer Staging (Seventh Edition). Single LR was noticed in 38 patients (61.3%), while multiple LR was observed in 24 patients (38.7%).

Full table
Comparison of SCC-Ag, CYFRA21-1 and CEA in normal individuals and EC patients with postoperative LR
Remarkable elevation was noticed in the SCC-Ag and CYFRA21-1 in patients with postoperative LR compared with those of the normal individuals (t=4.79, P<0.05; t=7.86, P<0.05, Table 2). Whereas, no statistical difference was noticed in the CEA level in patients with postoperative LR compared to that of the normal individuals (P>0.05, Table 2). Besides, the positive rates of SCC-Ag and CYFRA21-1 in the patients were 67.7% and 69.4%, which were higher than that of the CEA (16.1%), respectively (χ2=33.92, P<0.05; χ2=35.89, P<0.05, Table 2). Compared with the SCC-Ag, no statistical difference was observed in the positive rate of CYFRA21-1 in the patients with postoperative LR (Table 2).
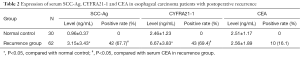
Full table
Correlation between pathological stage and serum SCC-Ag, CYFRA21-1 and CEA in EC patients with postoperative LR
To investigate the correlation between the postoperative pathological stage and serum tumor markers in these patients, correlation analysis was performed. Our data indicated the pathological stages were closely correlated to the serum SCC-Ag and CYFRA21-1. To be exact, in the patients with postoperative LR, the serum SCC-Ag and CYFRA21-1 were obviously higher in the patients of stage III compared to those of the stage I (SCC-Ag: t=3.442, P<0.05; CYFRA21-1: t=4.91, P<0.05, Table 3). Moreover, the serum CYFRA21-1 in patients of stage III was remarkably elevated compared to that in patients of stage II (t=2.31, P<0.05, Table 3). However, serum CEA showed no difference in patients of stage III compared to that of stage I and II, respectively. This indicated CEA was not correlated to the pathological stage of patients with postoperative LR.
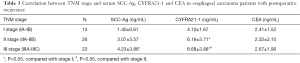
Full table
Changes of SCC-Ag, CEA and CYFRA21-1 after radiotherapy or chemoradiotherapy in EC patients with postoperative LR
In this section, we aim to investigate the changes of SCC-Ag, CEA and CYFRA21-1 after radiotherapy or chemoradiotherapy. Compared with the baseline levels, serum SCC-Ag and CYFRA21-1 were significantly decreased after treatment (SCC-Ag: t=3.24, P<0.05; CYFRA21-1: t=3.79, P<0.05). On the contrary, no difference was noticed in the serum CEA after treatment compared to that of the baseline levels (Table 4).

Full table
Survival analysis
The median survival duration for the 62 EC patients with postoperative LR was 12 months after radiotherapy or chemoradiotherapy (95% CI, 8.98–15.02). The survival rates at 1, 2, 3 and 5 years were 46.1%, 19.7%, 12.3%, and 8.2%, respectively (Figures 1A).
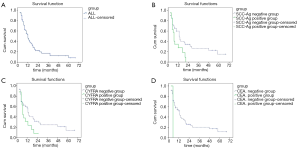
Among the EC patients with postoperative LR before radiotherapy or chemoradiotherapy, the median survival duration for the patients of SCC-Ag negative group was 20 months (95% CI, 9.9–30.09), while that for the SCC-Ag positive group was 8 months (95% CI, 4.7–11.30). As shown in Figure 1B, remarkably difference was noticed in the survival of SCC-Ag negative group compared to that of SCC-Ag positive group (χ2=5.918, P<0.05). The median survival duration for the patients of CYFRA21-1 negative group was 18 months (95% CI, 15.18–20.83), which was superior to the serum CYFRA21-1 positive group with a median survival duration of 8 months (95% CI, 4.55–11.45) (χ2=4.921, P<0.05, Figure 1C). Compared to the CEA negative group, the median survival duration showed no statistical difference in the CEA positive group [CEA negative group: 12 months (95% CI, 8.35–15.65); CEA positive group: 12 months (95% CI, 7.35–16.65)] (χ2=0.000, P>0.05, Figure 1D).
Among the EC patients with postoperative LR after radiotherapy or chemoradiotherapy, the median survival duration for the patients of SCC-Ag negative group was 13 months (95% CI, 9.28–16.72), while that for the SCC-Ag positive group was 7 months (95% CI, 5.70–8.31). As shown in Figure 2A, remarkably differences were noticed in the survival of SCC-Ag negative group compared to that of SCC-Ag positive group (χ2=5.30, P<0.05). Compared to the CYFRA21-1 negative group, the median survival duration showed no statistical difference in the CYFRA21-1 positive group [CYFRA21-1 negative group: 13 months (95% CI, 10.82–15.18); CYFRA21-1 positive group: 7 months (95% CI, 5.71–8.29)] (χ2=0.3.47, P>0.05, Figure 2B). Compared to the CEA negative group, the median survival duration showed no statistical difference in the CEA positive group [CEA negative group: 12 months (95% CI, 8.38–17.62); CEA positive group: 12 months] (χ2=0.14, P>0.05, Figure 2C).
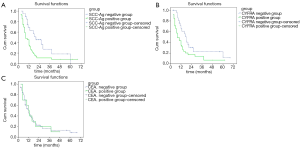
Identification of risk factors for overall survival in ESCC patients
In this study, multivariate Cox regression analysis was performed to identify the risk factors for overall survival in patients with postoperative LR, including age, gender, pathological stage, tumor lesions, SCC-Ag, CYFRA21-1 and CEA before and after radiotherapy or chemoradiotherapy. The results indicated no independent risk factors were identified (Table 5).
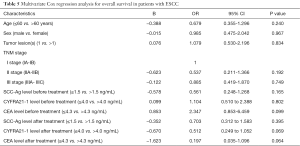
Full table
Discussion
Squamous cell carcinoma and adenocarcinoma consist of the major two type of EC, especially the squamous cell carcinoma with a prevalence of up to 90% in the area with high incidence of EC (4,11). SCC-Ag, a type of glycoprotein with a molecular weight of 48 kDa, was a subfraction of T4-A tumor antigen (12,13). It has been considered as a specific marker for the squamous cell cancer. According to the previous study, serum SCC-Ag was reported to be related to the tumor load and the activity of cancer cells (14). CYFRA21-1, a member of keratin localized in the squamous epithelial cells, was encoded by KRT19 gene. Generally, the expression of CYFRA21-1 was comparatively lower under normal conditions. However, in patients with squamous cell carcinoma, the cellular release of CYFRA21-1 was remarkably elevated, which resulted in the increase of CYFRA21-1 in the tissue fluid and blood (15,16). CEA, a member of immunoglobulin superfamily, has been frequently identified in the digestive system cancer originated from endoderm. Also, it plays important roles in the pathogenesis and metastasis of tumor by modulating the interaction between the cancer cells and the matrix (17,18).
Up to now, the positive rates of SCC-Ag, CYFRA21-1 and CEA about patients with EC varies in different studies. For example, Mao et al. revealed the positive ratios of serum CYFRA21-1 and CEA were 45.1% and 29.1% in 206 EC patients (19). Kawaguchi et al revealed the positive rate of serum CYFRA21-1 was 76.9% in ESCC patients (20). In a case report, Nozaki et al reported a case of ESCC patient with normal CEA and SCC-Ag (21). For the correlation between the tumor markers and postoperative LR, Brockmann et al. indicated elevation of serum CYFRA21-1 may serve as a predictor of postoperative LR of EC (22). Besides, Liu et al. revealed serum CEA improved the sensitivity to predict postoperative LR in these patients (23). Banki et al. reported normal serum CEA could not exclude the possibility of recurrent EC (24). In this study, most of the recurrent EC patients (96.8%) were diagnosed as esophageal squamous cell carcinoma, and the serum SCC-Ag and CYFRA21-1 were expressed in a majority of the recurrent patients with a ratio of 67.7% and 69.4%, respectively. The serum CEA in the recurrent patients showed no difference compared with that of the normal individuals. Meanwhile, we also determined their expression after radiotherapy or combination of radiotherapy and chemotherapy. Our results indicated that obvious decrease was noticed in the serum SCC-Ag and CYFRA21-1 after radiotherapy or combination of radiotherapy and chemotherapy compared with the baseline levels, except for CEA showed no difference. Taken together, it is reasonable to conclude that serum SCC-Ag and CYFRA21-1 may provide diagnostic value for the recurrent EC.
Among the patients received radical resection for treating EC, serum cancer biomarkers levels were reported to be related to the postoperative pathological stage. Simultaneously, the levels of serum SCC-Ag, CYFRA21-1 and CEA in late stage patients were higher compared to those of the early stage patients (19). To our best knowledge, a higher recurrence has been frequently reported in patients of late stage, especially those received no chemotherapy and/or radiotherapy. To date, it is still controversial on the correlation between serum cancer markers and the pathological stages, as well as the patients’ survival. For example, there was a tendency for higher serum CYFRA21-1 concentrations in advanced T-stage rather than N or M stages in esophageal patients (22). In addition, the positive rate of CYFRA21-1 increased with the progression of esophageal cancer with a ratio of 22.2% of pTNM stage 0-IIA and 77.8% pTNM stage IIB/III, however, SCC-Ag and CEA rates were not correlated to the pTNM stages. On this basis, the authors concluded that CYFRA21-1 elevation contributed to the diagnosis of recurrence in the absence of clinical data and imaging monitoring (20). In a previous study, Godfrey et al. used gel-based reverse transcription-PCR (RT-PCR) and Taqman quantitative RT-PCR for the determination of CEA mRNA in the lymph nodes obtained from EC patients. Kaplan-Meier analysis showed that CEA positivity resulted in significantly lower disease-free and overall survival compared to the CEA negativity (25). Setoyama et al. reported that CEA mRNA was identified in a majority of patients (76.5%) experiencing recurrence, while the positivity of CEA mRNA was correlated to the pathological TNM staging. Besides, patients positive for CEA mRNA showed significantly shorter disease-free interval compared to those with negative CEA mRNA (26). Furthermore, Mao et al. (19) revealed the serum SCC-Ag and CYFRA21-1 were closely related to the TNM staging in the EC patients. According to our data, serum SCC-Ag and CYFRA21-1 were correlated to the TNM staging in recurrent esophageal cancer patients. To be exact, the serum SCC-Ag and CYFRA21-1 in the stage III patients were obviously higher than those in patients of stage I. Similarly, the serum CYFRA21-1 in stage III patients was also higher than that in stage II patients. Nevertheless, no correlation was noticed between serum CEA and pathological TNM stage in recurrent EC. Our results indicate the monitoring of serum SCC-Ag and CYFRA21-1 is of prime importance in the advanced esophageal cancer patients after radical resection as the elevation of these markers contributes to the diagnosis of recurrence.
As previously described (27,28), part of the patients with LR of EC showed long-term survival after radiotherapy or chemoradiotherapy. In this study, the survival rates at 1, 2, 3 and 5 years were 46.1%, 19.7%, 12.3%, and 8.2%, which was in line with the previous report (29). This indicated that EC patients with LR may present long-term survival after positive treatment. In this study, the serum SCC-Ag and CYFRA21-1 showed obvious decrease after radiotherapy or chemoradiotherapy. Moreover, prior to any treatment for LR, the survival rates of patients with negativity of SCC-Ag and CYFRA21-1 were superior to these with positivity of these markers. Whereas, no statistical difference was noticed in the patients with positivity of CEA compared to those of negativity before radiotherapy or combination of chemotherapy and radiotherapy. After radiotherapy or chemoradiotherapy, the survival rates of patients with negativity of SCC-Ag were superior to these with positivity of the marker. No statistical difference was noticed in the survival duration of patients with negativity in CYFR21-1 and CEA after treatment compared with those with positivity. Meanwhile, no statistical difference was noticed in the survival duration after treatment for the CYFR21-1, while remarkable difference was noticed before treatment. This may be related to the fact that no obvious decrease was noticed in the CYFR21-1 one month after treatment, and the small sample size. Cox regression analysis was performed to identify the risk factors for the survival, and no risk factors were found. We speculated that this may be associated with the small sample size. In future, studies of large sample size are needed to investigate the risk factors of EC with LR.
In conclusion, serum SCC-Ag and CYFRA21-1 contribute to the diagnosis of postoperative LR in EC patients. In addition, radiotherapy and/or chemotherapy is beneficial to the extension of overall survival in these patients.
Acknowledgments
Funding: This project is sponsored by the Changzhou Municipal Health Bureau Instructional Science and Technology Plan (No. WZ201018), Changzhou Municipal “831” Scientific Research Program, Changzhou Municipal Medical Innovation Talent Program (No. 2-14), and Jiangsu Provincial “333 Talent” Program.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.49). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocols were approved by the Ethic Committee of Changzhou No. 2 People’s Hospital (Approval No.: 2015 RES 011-01). The participants of the present study did not write informed consent before taking part since this was a retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Scarpa ES, Ninfali P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. Int J Mol Sci 2015;16:15727-42. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Rouvelas I, Zeng W, Lindblad M, et al. Survival after surgery for oesophageal cancer: a population-based study. Lancet Oncol 2005;6:864-70. [Crossref] [PubMed]
- Guo XF, Mao T, Gu ZT, et al. Clinical study on postoperative recurrence in patients with pN0 esophageal squamous cell carcinoma. J Cardiothorac Surg 2014;9:150. [Crossref] [PubMed]
- Zhang J, Zhu Z, Liu Y, et al. Diagnostic value of multiple tumor markers for patients with esophageal carcinoma. PLoS One 2015;10:e0116951 [Crossref] [PubMed]
- Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol 2005;129:118-29. [Crossref] [PubMed]
- Zheng X, Xing S, Liu XM, et al. Establishment of using serum YKL-40 and SCCA in combination for the diagnosis of patients with esophageal squamous cell carcinoma. BMC Cancer 2014;14:490. [Crossref] [PubMed]
- Inghirami G, Zhu BY, Chess L, et al. Flow cytometric and immunohistochemical characterization of the gamma/delta T-lymphocyte population in normal human lymphoid tissue and peripheral blood. Am J Pathol 1990;136:357-67. [PubMed]
- Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer 2005;113:456-63. [Crossref] [PubMed]
- Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer 1977;40:1621-8. [Crossref] [PubMed]
- Hung CM, Chang CC, Lin CW, et al. Cucurbitacin E as inducer of cell death and apoptosis in human oral squamous cell carcinoma cell line SAS. Int J Mol Sci 2013;14:17147-56. [Crossref] [PubMed]
- Micke O, Bruns F, Schäfer U, et al. The impact of squamous cell carcinoma (SCC) antigen in patients with advanced cancer of uterine cervix treated with (chemo-)radiotherapy. Anticancer Res 2005;25:1663-6. [PubMed]
- Edelman MJ, Hodgson L, Rosenblatt PY, et al. CYFRA 21-1 as a prognostic and predictive marker in advanced non-small-cell lung cancer in a prospective trial: CALGB 150304. J Thorac Oncol 2012;7:649-54. [Crossref] [PubMed]
- Schweizer J, Bowden PE, Coulombe PA, et al. New consensus nomenclature for mammalian keratins. J Cell Biol 2006;174:169-74. [Crossref] [PubMed]
- Holmes A, Abraham DJ, Sa S, et al. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem 2001;276:10594-601. [Crossref] [PubMed]
- Gillette EL, Mahler PA, Powers BE, et al. Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys 1995;31:1309-18. [Crossref] [PubMed]
- Mao YS, Zhang DC, Zhao XH, et al. Significance of CEA, SCC and Cyfra21-1 serum test in esophageal cancer. Zhonghua Zhong Liu Za Zhi 2003;25:457-60. [PubMed]
- Kawaguchi H, Ohno S, Miyazaki M, et al. CYFRA 21-1 determination in patients with esophageal squamous cell carcinoma: clinical utility for detection of recurrences. Cancer 2000;89:1413-7. [Crossref] [PubMed]
- Nozaki Y, Nishida T, Hori Y, et al. Chemoradiotherapy is effective for primary esophageal adenosquamous cell carcinoma but ineffective for the metastatic adenocarcinoma component. Nihon Shokakibyo Gakkai Zasshi 2015;112:278-86. [PubMed]
- Brockmann JG, St Nottberg H, Glodny B, et al. Analysis of serum CYFRA 21-1 concentrations in patients with esophageal cancer. Anticancer Res 2000;20:4899-904. [PubMed]
- Liu Z, Jiang M, Yan F, et al. Multipoint quantification of multimarker genes in peripheral blood and micrometastasis characteristic in peri-operative esophageal cancer patients. Cancer Lett 2008;261:46-54. [Crossref] [PubMed]
- Banki F, Yacoub WN, Hagen JA, et al. Plasma DNA is more reliable than carcinoembryonic antigen for diagnosis of recurrent esophageal cancer. J Am Coll Surg 2008;207:30-5. [Crossref] [PubMed]
- Godfrey TE, Raja S, Finkelstein SD, et al. Prognostic value of quantitative reverse transcription-polymerase chain reaction in lymph node-negative esophageal cancer patients. Clin Cancer Res 2001;7:4041-8. [PubMed]
- Setoyama T, Natsugoe S, Okumura H, et al. Carcinoembryonic antigen messenger RNA expression in blood predicts recurrence in esophageal cancer. Clin Cancer Res 2006;12:5972-7. [Crossref] [PubMed]
- Bao Y, Liu S, Zhou Q, et al. Three-dimensional conformal radiotherapy with concurrent chemotherapy for postoperative recurrence of esophageal squamous cell carcinoma: clinical efficacy and failure pattern. Radiat Oncol 2013;8:241. [Crossref] [PubMed]
- Kobayashi R, Yamashita H, Okuma K, et al. Salvage radiation therapy and chemoradiation therapy for postoperative locoregional recurrence of esophageal cancer. Dis Esophagus. 2014;27:72-8. [Crossref] [PubMed]
- Ma DY, Tan BX, Liu M, et al. Concurrent three-dimensional conformal radiotherapy and chemotherapy for postoperative recurrence of mediastinal lymph node metastases in patients with esophageal squamous cell carcinoma: a phase 2 single-institution study. Radiat Oncol 2014;9:28. [Crossref] [PubMed]


