
Fusobacterium nucleatum infection is correlated with tumor metastasis and postoperative survival of colorectal cancer patients in China
Introduction
Fusobacterium nucleatum (F. nucleatum), a gram-negative anaerobic species of the Bacteroidaceae family, is one of the most common species of bacteria isolated from dental plaque biofilms. This species has been found to play important roles in pericarditis, appendicitis and several oral diseases (1). For example, F. nucleatum can secrete butanoic acids and lipopolysaccharide, which has been proved to inhibit human T-cell responses to mitogens and antigens (2) and to induce apoptotic cell death in peripheral blood mononuclear cells (PBMCs) (3). Moreover, all strains of F. nucleatum that have been tested can stimulate epithelial cells to secrete proteolytic enzymes, including Matrix metalloproteinases (MMPs) such as MMP-13, which plays an important part in extracellular matrix (ECM) degradation (4).
Colorectal carcinoma (CRC) is the third leading cause of cancer death in America and the second most prevalent cancer in China (5). The pathogenesis of sporadic CRC remains unclear. Recent studies have shown that the F. nucleatum infection rate (prevalence) and infection load (abundance) was higher in CRC than in matched normal tissue from western patients (6,7). In addition, a strain of F. nucleatum isolated directly from a CRC sample could invade human colonic epithelial cells (6,7).
Progression and survival of CRC patients is dependent on the complex interaction between tumor and host. The pronounced local inflammatory response intra-tumor and around the tumor is thought to represent the anti-tumor immune response. There has been increasing interest in establishing the cellular composition of immune cell infiltrates and their relationship with survival in CRC patients, and over 140 studies have proved that the number/density of immune cells intratumor and around the tumor is an important hint for prognostics, which may be incorporated into routine clinical assessment. In tumor stroma, inflammatory cell types are mainly T lymphocyte and their subsets, and they are collectively known as the tumor infiltrating lymphocytes (TIL). The density of memory T-lymphocytes (CD45RO positive) can reflect local immune response, and the increasing densities of these long-lived memory T-lymphocytes at both tumor centre and invasive margin was associated with improved survival or reduced metastatic potential (8-13). T regulatory lymphocytes (Treg cells, FOXP3 positive) play a role in the suppression of immune responses to foreign and self-antigenic material, and two studies have reported that high density of Treg cells was a strong prognostic factor associated with poorer survival in primary operable CRC (9,14,15). To date, the prevalence of F. nucleatum in CRC patients from Asian, including China, has not been determined, and the biological characteristics of F. nucleatum has not been investigated in CRC patients. We therefore assayed tumor and matched normal tissue from 152 CRC patients for the infection rate and infection load of F. nucleatum. In order to evaluate the biological impact of this infection, we assayed the abundance of memory and regulatory T lymphocytes in these samples to examine the immune response in primary operable CRC. In addition, we evaluated the expression of the proteolytic enzymes MMP9 and MMP11 to identify whether F. nucleatum infection in CRC has a similar role in oral disease.
Methods
Patients and samples
Fresh colorectal adenocarcinoma and corresponding adjacent normal tissues were obtained at surgery from 152 patients who underwent radical surgery at Peking University Cancer Hospital, Beijing, China, between January 2009 and December 2010. All these patients were diagnosed as CRC, and did not receive chemotherapy or radiotherapy before surgery. In addition, for these patients, there is no evidence of concomitant infectious or immunologic diseases and no infection after surgery. These patients included 95 males (62.5%) and 57 females (37.5%), with a median age of 62 years (range, 28–84 years; Table 1). Normal tissues (NT) were obtained at least 5.0 cm from the tumor margin, and were confirmed as negative for tumor by histological examination. These patients were followed-up postoperatively, over a period of at least 5 years.
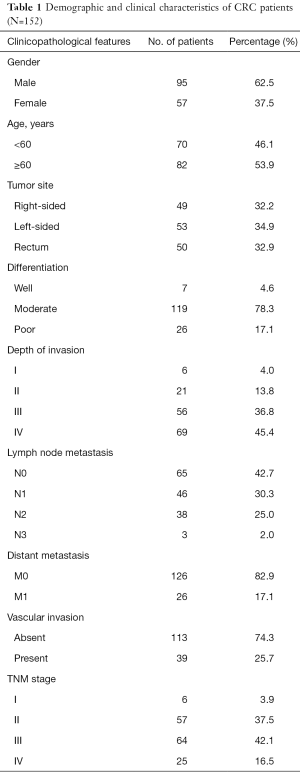
Full table
All specimens, obtained immediately after surgical resection, were snap-frozen in liquid nitrogen and stored at −80 °C. Sixteen samples used for immunohistochemistry must reach the following requirements: (I) moderately differentiated adenocarcinoma; (II) T3N0M0 stage; (III) F. nucleatum infection can be detected in both tumor tissue and NT; (IV) age gap between these 16 patients is less than 5 years (57–60 years).
All clinicopathological features were obtained from patient records. Overall survival (OS) or follow-up time was calculated from the date of surgery to date of death or last follow-up respectively. This study was approved by the institutional review board of Peking University Cancer Hospital (Ethical approval number: 2008110621), and written informed consent was acquired from each patient.
Isolation of genomic DNA
DNA was extracted from tissue samples using Transgene® Easy Pure genomic DNA Kits, according to the manufacturer’s instructions. DNA concentration was assayed by Nanodrop 2000 (Thermo scientific). The quality of each DNA sample was examined by PCR, using the human β-actin gene primers 5'-TTAGTTGCGTTACACCCTTTC-3' (forward) and 5'-ACCTTCACCGTTCCAGTTT-3' (reverse), resulting in a 150 bp product.
Cloning a fragment of F. nucleatum nusG gene to prepare loading controls
We randomly evaluated several DNA samples by PCR and chose one sample positive for F. nucleatum infection. The PCR product was confirmed by gene sequencing, then amplified and cloned into a plasmid using the TA cloning technique to construct a plasmid containing the 161-bp fragment of the F. nucleatum nusG gene. The copy number of the plasmid was calculated according to the formula: copy number=(amount×6.022×1023)/(length×1×109×650). The plasmid was diluted to 108~101 copies/µL and used as loading controls.
Quantitative real-time PCR to evaluate F. nucleatum infection
The infection rate and infection load of F. nucleatum (ATCC 25586) in samples from CRC patients were analyzed by quantitative real-time PCR, using the primers 5'-CAACCATTACTTTAACTCTACCATGTTCA-3' (forward) and 5'-GTTGACTTTACAGAAGGAGATTATGTAAAAATC-3' (reverse). The resulting 161-bp product matched a sequence of the nusG gene (GenBank accession AAL94126.1) of F. nucleatum, but did not match any sequence in other genes of any other species.
Quantitative real-time PCR was conducted using the ABI 7500 Sequence Detection System (Applied Biosystems). Each 20 µL aliquot of the reaction mixture contained 20 ng DNA, 1.5 µM of each primer, and 10 µL Power SYBR® Green PCR Master Mix (Applied Biosystems Inc., California, USA). The amplification protocol consisted of an initial denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min, and extension at 72 °C for 15 s. All samples were assayed in triplicate.
Immunohistochemistry
Formalin-fixed, paraffin-embedded CRC and adjacent non-tumor tissue samples were sectioned at 4 µm thickness, deparaffinized, and incubated with 3% hydrogen peroxide for 10 min to inhibit endogenous peroxidases. To assay for MMP9, CD45RO and FOXP3, sections were heated in a microwave oven for antigen retrieval; to assay for MMP11, sections were heated in an autoclave. The samples were incubated with 5% nonfat milk to prevent nonspecific binding and overnight at 4 °C with primary antibodies to MMP9 (rabbit monoclonal antibody, 1:200; CST, Boston, MA), MMP11 (mouse monoclonal antibody, 1:250; Abcam, Cambridge, MA), CD45RO (mouse monoclonal antibody, 1:50; Novus, Littleton, NH), and FOXP3 (mouse monoclonal antibody, 1:100; Abcam). Staining results were evaluated by two independent pathologists who were blind to the clinical data pertaining to the patients For MMP9 and MMP11, samples with 0–5%, 6–25%, 26–50%, 51–75%, and >75% positive cells were scored as −, +, ++, +++, and ++++, respectively. Expression of CD45RO and FOXP3 was assessed by counting positive lymphocytes in five high-power fields and calculating their mean values.
Quality control
DNA preparation and PCR amplification was conducted at different locations within the laboratory, with specimens moving through the laboratory in only one direction. Environmental contamination was monitored consistently before each experiment, and laboratory areas were decontaminated regularly. To monitor potential contamination during DNA preparation, DNA was prepared from a colorectal tissue sample of a nude mouse after each batch of 6 pairs of human CRCs and adjacent NT samples. To confirm absence of contamination during DNA preparation, human β-actin and nusG gene of F. nucleatum were tested in these control samples.
Each 96-well plate used for real time-PCR contained several control samples, including three nude mouse colorectal DNA samples, processed along with the human specimens, as DNA preparation controls; four samples from which DNA template had been omitted; and, as positive controls, plasmids containing 109~102 copies of nusG gene DNA.
Statistical analysis
The difference in infection rates between tumor and matched NT were analyzed using the Chi-square test, whereas the difference in copy number was analyzed using the rank sum test. Univariate and multivariate logistic regression analyses were performed to estimate the odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for associations between the presence of F. nucleatum DNA and clinicopathological features. CD45RO+ lymphocyte counts in normal and tumor tissues were compared using paired-samples t-tests, CD45RO+ lymphocyte counts in the high and low F. nucleatum copy groups were compared using independent-samples t-tests, and FOXP3+ lymphocyte counts were compared using the rank sum test. Expression of MMP9 and MMP11 in normal and carcinoma tissues, and in high and low-copy groups, was compared using Chi-squared tests. OS was analyzed using the Kaplan-Meier model and compared using the log-rank test. All statistical analyses were performed using SPSS 16.0 statistical software (SPSS, Chicago, IL, USA), with P<0.05 considered statistically significant.
Results
Infection rate and infection load of F. nucleatum infection in CRC patients were higher than in matched NT
F. nucleatum was detected in 118 of the 152 (77.6%) tumor tissues and in 87 of the 152 (57.2%) matched NT (P<0.001). Infection load (copy numbers of infection) ranged from 1 to 240,418 (median, 87) in the CRC tissues and from 2 to 3,685 (median, 37) in adjacent NT (P<0.001). In 84 of 152 patients (55.3%), F. nucleatum was detected in both the tumor and adjacent NT, with median copy numbers of 173 and 38, respectively. In 68 of these 84 patients, the copy number was higher in tumor tissues than in matched NT (P<0.001; Table 2).
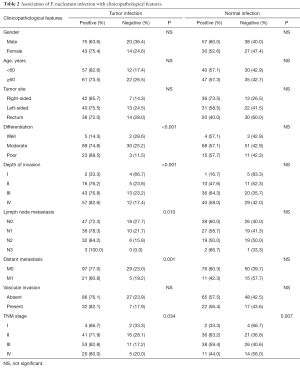
Full table
Correlation of F. nucleatum infection with clinicopathological features
F. nucleatum infection of tumor tissue was related with poorer tumor differentiation (P<0.001), advanced invasion depth (P<0.001), lymph node metastasis (p=0.01), distant metastasis (P=0.001) and TNM stage (P=0.034), whereas infection of NT was only associated with TNM stage (P=0.007). For detailed data, please see Table 2. Using the median number of infectious particles in tumor tissue samples, we classified the 84 pairs of samples in which both tumor tissue and matched NT were positive for F. nucleatum into high- and low-abundance group. Then we analyzed the correlation between F. nucleatum copy number and patients’ clinicopathological features and found that high-copy infection was associated only with tumor stage (P=0.023).
Immunohistochemical evaluation
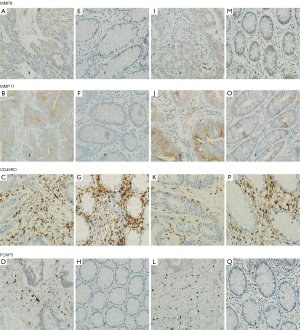
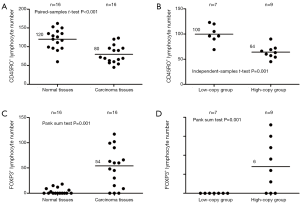
In order to explore whether F. nucleatum infection can affect the biological behavior of host cells and tumor cells, we assayed MMP9, MMP11, CD45RO and FOXP3 expression in 16 pairs of T3N0M0 tumor and matched NT that were both infected with F. nucleatum. Expression of MMP9 was observed in the nucleus and the cytoplasm. MMP11 showed weak to moderate cytoplasmic staining, and extracellular material was often distinctly stained. CD45RO was observed in the cytoplasm or on the plasmalemma of lymphocytes, and FOXP3 was observed in the nucleus of lymphocytes (Figure 1). MMP9, MMP11 and FOXP3 were highly expressed in tumor tissues, and CD45RO was highly expressed in NT (Table 3). When we classified these 16 pairs of samples according to the median number of F. nucleatum copies in the tumor samples, we found that the mean number of lymphocytes positive for CD45RO was significantly higher in the low-copy group than in the high-copy group (120 vs. 81, P=0.001), whereas the mean number of lymphocytes positive for FOXP3 was significantly higher in the high-copy than in the low-copy group (54 vs. 3, P=0.001; Figure 2).

Full table
Survival analysis
Using the median number [87] of F. nucleatum copies in tumor tissues as a cut off value, we classified all 118 patients positive for tumor infection into a high- and a low-copy group, and compared OS after surgery in these two groups. Of the 59 patients in the low-copy group, 14 died, and median OS was 39.5 months. Of the 57 patients in the high-copy group, 24 died, and the median OS was 32.7 months. The remaining 2 patients were lost to follow-up and the rate was 1.7%. Kaplan-Meier survival analysis and Log-rank test showed that patients in the high-copy group had a shorter postoperative survival than the low-copy group (P=0.027, Figure 3).
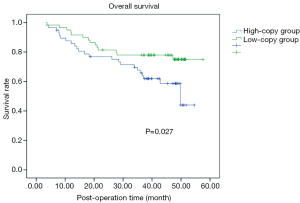
Next, we included other clinicopathological factors in the Cox model for multivariate survival analysis. In the total patients, we have found that tumor stage was an independent prognostic factor of postoperative survival in these CRC patients, that is, advanced stage was correlated poorer prognosis (P=0.018), however, other factors (including F. nucleatum infection) were not independent prognostic factors. Furthermore, we evaluated the effect of various factors on postoperative survival in the subset of CRC patients with F. nucleatum infection, and found that tumor stage and F. nucleatum infection were independent prognostic factors, that is, advanced stage and high-load infection were correlated poorer prognosis (P=0.023 and 0.032, respectively).
Discussion
Infection with microorganisms may play an important role in tumor development and progression. For example, HPV is involved in cervical cancer (16), EBV in nasopharyngeal carcinoma (17), Helicobacter pylori in gastric cancer (18), and HBV in hepatocellular carcinoma (19). F. nucleatum was detected in the lamina propria of tumor tissues in CRC patients, wherein infection rate and infection load was higher in tumors than in adjacent NT (6,7). These findings suggested that F. nuleatum may be closely associated with CRCs (20). Since these previous studies involved western patients, we assessed the infection rate and infection load of F. nucleatum in CRC patients in China, and evaluated the potential effect of this bacterium on the hosts.
Using strict inclusion criteria, we chose 152 CRC patients with complete clinical and pathological data who underwent radical resection. Using quantitative PCR, we determined the copy number of F. nucleatum DNA in samples and found that both F. nucleatum infection rate and copy number were significantly higher in tumor tissues than in adjacent NT (6,7). We also assessed the association of F. nucleatum infection with clinicopathological features and found that F. nucleatum infection of tumor tissue was related with poorer tumor differentiation (P<0.001), advanced invasion depth (P<0.001), lymph node metastasis (P=0.01), distant metastasis (P=0.001) and TNM stage (P=0.034). depth of tumor invasion (P=0.039), whereas infection of NT was only associated with TNM stage (P=0.007). In addition, when we compared the clinicopathological features in patients with high- and low-copy numbers of F. nucleatum, we found that high-copy infection was only correlated with tumor stage (P=0.023). Previous studies also have shown correlations of F. nucleatum abundance with patient geographic location (7) and number of metastatic lymph nodes (6). Survival analysis showed that patients in the high-copy group had shorter survival after surgery than the low-copy group (P=0.027). These findings suggested that F. nucleatum might play a role in progression and prognosis of CRC patients.
F. nucleatum can stimulate locally infected epithelial cells to secrete MMPs and promote the apoptosis of immune cells (4). In our study, these characteristics were assessed in CRC patients by immunohistochemical assays of MMP9, MMP11, CD45RO and FOXP3 in tumor tissues and corresponding NT. To minimize confounding factors, we selected 16 patients of T3N0M0 tumor staging, with both tumor and matched NT being infected with F. nucleatum. We found that, compared with specimens with low infection-load, those with high infection-load contained fewer CD45RO+ cytotoxic T lymphocytes (21), which can secrete cytokines involved in immune function and kill viruses and tumor cells, thus constituting an important defense in anti-viral and anti-tumor immunity. Therefore, a reduction in the number of CD45RO+ may reflect an inactive local immune status. In contrast, specimens in the high-abundance group contained more T regulatory lymphocytes, as determined by FOXP3+ expression (21), than the low-abundance group. These findings also indicated that localized F. nucleatum infection in CRC patients may play a role in immune suppression. There was no difference in MMP9 and MMP11 expression between these two groups, which might be due to the complex mechanism by which the secretion of MMPs is regulated. Moreover, the relatively small number of patient sample assayed immunohistochemically was insufficient to rule out confounding factors.
The mechanisms by which F. nucleatum caused CRC have been elucidated in recent years. F. nucleatum can generate a proinflammatory microenvironment that is conducive for colorectal neoplasia progression through recruitment of tumor-infiltrating immune cells (22). In addition, another study also demonstrated that F. nucleatum can adhere to, invade, and induce oncogenic and inflammatory responses to stimulate growth of CRC cells through its unique FadA adhesin (23). So in future study, we will also evaluate the expression level of FadA adhesin, and also will explore the possible correlation of FadA adhesin with other markers of CRC or with clinicopathological factors and prognosis of CRC patients.
To our knowledge, this is the first study to assess the effect of F. nucleatum infection in CRC patients in Chian, both at the systemic and local levels. We used ATCC 25,586 type-specific primers for F.N. q-PCR, which might have underestimated the total infection rate. Another limitation was the lack of normal colorectal mucosa specimens from healthy individuals, and this may be remedied by cooperating with our endoscopic department in the future.
Conclusions
Our results have provided evidence that F. nucleatum may act as a pathogenic factor in CRC patients, which may lead to improvement in the prevention, diagnosis and treatment of CRC. F. nucleatum infection was correlated with tumor metastasis and postoperative survival of CRC patients.
Acknowledgments
Funding: This work was supported by the Capital Health Research and Development of Special Funds (approval No.: 2016-2-2151); Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (approval No.: XMLX201708); National Natural Science Funding (approval No.: 81272765, 61372028, and 61571437); and Capital Characteristic Clinical Application Research (approval No.: Z161100000516065).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.45). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Peking University Cancer Hospital (Ethical approval number: 2008110621), and written informed consent was acquired from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huynh T, Kapur RV, Kaplan CW, et al. The role of aggregation in Fusobacterium nucleatum- induced immune cell death. J Endod 2011;37:1531-5. [Crossref] [PubMed]
- Shenker BJ, DiRienzo JM. Suppression of human peripheral blood lymphocytes by Fusobacterium nucleatum. J Immunol 1984;132:2357-62. [PubMed]
- Jewett A, Hume WR, Le H, et al. Induction of apoptotic cell death in peripheral blood mononuclear and polymorphonuclear cells by an oral bacterium, Fusobacterium nucleatum. Infect Immun 2000;68:1893-8. [Crossref] [PubMed]
- Signat B, Roques C, Poulet P, et al. Fusobacterium nucleatum in periodontal health and disease. Curr Issues Mol Biol 2011;13:25-36. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012;22:299-306. [Crossref] [PubMed]
- Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 2012;22:292-8. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Lee WS, Park S, Lee WY, et al. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer 2010;116:5188-99. [Crossref] [PubMed]
- Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol 2010;222:350-66. [Crossref] [PubMed]
- Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005;353:2654-66. [Crossref] [PubMed]
- Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009;27:5944-51. [Crossref] [PubMed]
- Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011;29:610-8. [Crossref] [PubMed]
- Sinicrope FA, Rego RL, Ansell SM, et al. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 2009;137:1270-9. [Crossref] [PubMed]
- Suzuki H, Chikazawa N, Tasaka T, et al. Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother 2010;59:653-61. [Crossref] [PubMed]
- Bosch FX. Human papillomavirus: science and technologies for the elimination of cervical cancer. Expert Opin Pharmacother 2011;12:2189-204. [Crossref] [PubMed]
- Fang CY, Huang SY, Wu CC, et al. The synergistic effect of chemical carcinogens enhances Epstein-Barr virus reactivation and tumor progression of nasopharyngeal carcinoma cells. PLoS One 2012;7:e44810 [Crossref] [PubMed]
- Li Z, Chen D, Zhang C, et al. HLA polymorphisms are associated with Helicobacter pylori infected gastric cancer in a high risk population, China. Immunogenetics 2005;56:781-7. [Crossref] [PubMed]
- Kim BK, Han KH, Ahn SH. Prevention of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Oncology 2011;81:41-9. [Crossref] [PubMed]
- Ray K. Colorectal cancer: Fusobacterium nucleatum found in colon cancer tissue--could an infection cause colorectal cancer? Nat Rev Gastroenterol Hepatol 2011;8:662. [Crossref] [PubMed]
- Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev 2012;38:451-66. [Crossref] [PubMed]
- Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013;14:207-15. [Crossref] [PubMed]
- Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013;14:195-206. [Crossref] [PubMed]


