
PIK3CA mutated, hormonal receptors and HER2: individual targets but partnered in the escape to targeted therapy in breast cancer
Introduction
Breast cancer is the most frequent cancer in women worldwide (1). Several discoveries in the biology of this malignancy leaded to the design of anti-endocrine and targeted therapy that improved significantly the outcome of these tumors while several resistance mechanisms have been unveiled.
PIK3CA mutation is a well-known resistance mechanism with high prevalence in breast cancer. In a recent work, Loibl et al. [2016] described in a pool of 967 HER2+ breast cancer patients treated with neoadjuvant anti-HER2 therapy and chemotherapy that PIK3CA mutations were related with a significantly lower pathological complete response (pCR) rates (16.2% vs. 29.6%; P<0.001) (2). The subgroups analysis in this work showed statistical trends in the association with distant metastases-free survival in estrogen receptor-positive patients regardless their pathological response where PIK3CA mutations confers a worse outcome (P=0.0502). This study raises the question about the better strategies to manage HER2+/hormonal receptors positive breast tumors with PIK3CA mutations and about the benefit for these patients from targeted therapy with HER2 as the sole target.
It’s well had known that PI3K pathway activation is a canonical pathway in several cancer types and a mechanism of resistance to anti endocrine therapy in ER+ breast cancer and also it provides resistance to trastuzumab treatment in HER2 tumors (3,4). Importance of PIK3CA in breast cancer biology has been described by several genomic projects.
PIK3CA mutations in HER2 tumors confers resitance to anti-HER2 targeted therapy
Data from TCGA describe a frequency of 36% of mutations were missense mutations are the most frequent (Figure 1) (5). Frequency of PIK3CA mutations in the molecular subtypes of breast cancer is, luminal A, 45%; luminal B, 29%; HER2 enriched, 39% and basal-like, 9%. There are three hotspots PIK3CA mutations (≈80%) in the regions encoding the helical (E542K, E545K) or catalytic (H1047R) domains of p110α (6). Data from several populations suggests that frequency of PIK3CA mutations is not related to ethnicity but distribution of breast cancer subtypes (7).
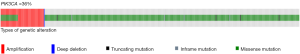
PIK3CA mutations have a driver role in breast cancer (8). This gene encodes the protein p110 that participate in signal transduction of mitogenic signals, have interaction and share pathways with important drivers in breast cancer biology (Figure 2). In vitro studies have shown that a small increase in the expression of PIK3CA wild type is sufficient to confer resistance to trastuzumab while HER2 cell lines harboring PIK3CA mutations are resistant to trastuzumab alone and in combination with lapatinib or pertuzumab (4,9).
Biomarkers evaluation in the most important trials with anti-HER2 targeted therapy leaded to the association of lower response rates in patients with PIK3CA mutated. In the NeoALTTO trial, differences in pCR were highly significant (53.1% vs. 28.6%, for PIK3CA wild type vs. mutated, respectively; P=0.012) (10).
In the pooled analysis by Loibl et al. [2016], there were not differences in rates of pCR in the subgroup of HR− patients (36.4% vs. 27.2%; P=0.125, for PIK3CA wt vs. mutant, respectively); however, this difference was highly significant in HR+ patients (24.2% vs. 7.6%; P<0.0.001, for PIK3CA wt vs. mutant, respectively). Interestingly, when comparison was done according to treatment, there were differences in the group of patients receiving trastuzumab plus lapatinib. There was observed a slight advantage in terms of disease-free survival in patients with PIK3CA wild type (P=0.0502) (2).
The lack of prognostic significance of PIK3CA mutations in hormone receptor negative patients could be explained by the types of mutations. In the study of Pereira et al., evaluating 2,433 breast cancer patients, it was observed a significant enrichment of mutations in codons 345, 542 and 545 in estrogen receptor positive tumors while in estrogen negative, mutations in codon 1047 were most frequent (11).
In the other hand, PI3K inhibition increase ER expression and tumor cells trend to adopt patterns of luminal A gene expression, for this reason is a good idea to combine anti-endocrine therapy with PI3K inhibitors in order to overcome the resistance (12).
What is the value of pCR in the outcome of HER2+/HR+ breast cancer?
pCR as surrogate of disease-free or overall survival (OS) is controversial although is frequently used as endpoint for approval in clinical trials. A meta-regression of 29 randomized neoadjuvant trials described only a weak association between odds ratios with hazard ratios for disease-free or OS (13).
It’s well known that luminal tumors achieve low rates of pCR after the neoadjuvant chemotherapy and this not influence the outcome (14). In regard to HER2+ tumors treated with anti-HER2 targeted therapy, data from the NeoALTTO study showed that pCR is associated to a better event-free survival only in the group of hormone receptors negative patients (HR =0.34; 95% CI: 0.17–0.63; P=0.001) in contrast to hormone receptors positive patients where significant differences were not observed (HR =0.50; 95% CI: 0.18–1.13; P=0.13) (15). In a pooled analysis of 11,955 patients from 12 neoadjuvant trials, the subgroup analysis show no improving in OS in HER2 positive, hormone-receptor positive patients treated or not with trastuzumab (HR =0.56, 95% CI: 0.23–1.37 and HR =0.57, 95% CI: 0.31–1.04), although the analysis in all HER2+/hormonal receptors-positive patients, the benefit for patients achieving pCR was evident (16).
Future perspectives
HER2-positive breast cancer corresponds to a superfamily of breast tumors (as coined by von Minckwitz) with great molecular complexity (17). Although expression of hormone receptors in HER2 tumors is associated with better outcome, mechanisms of resistance could be developed compromising the efficacy of the targeted therapy (18). PIK3CA mutations are one of these mechanisms.
Several in vitro and in vivo studies have shown that use of combined therapy targeting MAPK and HER2 could be an effective treatment approach in patients HER2+/PIK3CA mutated whilst molecular data from clinical trials revealed the importance of to determine the mutational status of PIK3CA supported by the inclusion of PIK3CA/MTOR/AKT inhibitors in the therapeutic strategies (19).
Despite the evidence, there is questions regarding why ER-negative tumors seems more sensitive than ER-positive tumors to combination of anti-HER2 therapy and PIK3CA/MTOR/AKT inhibitors. In the BOLERO-1 trial of combination of everolimus (or placebo) with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer was observed a clinical benefit in the ER-negative group (although not statistically significant by parameters chosen by the investigators) (20). This finding was also corroborated in BOLERO-3 trial in women with trastuzumab and paclitaxel resistant breast cancer that were randomized to receive everolimus plus trastuzumab and vinorelbine or to placebo plus trastuzumab plus vinorelbine. The benefit for everolimus addition was seen in ER-negative patients. The biomarkers study in this trial showed a benefit in PTEN low and pS6 high tumors (21). In addition, PIK3CA mutations was not predictive for benefit to everolimus despite this mutation produce a phenotype similar to PTEN low, indicating additional mechanisms of resistance.
In the other hand, the BOLERO-2 trial showed that addition of anti-endocrine therapy to everolimus is better than only everolimus in hormone receptors-positive patients (22).
Future studies will add a better characterization of resistance mechanisms. Currently, there are 26 clinical trials in breast cancer with PIK3CA inhibitors registered in clinicaltrials.gov while basic research find new mechanisms of resistance such as PIM1, recently involved in resistance to PIK3CA inhibitors (23).
Although PIK3CA, HER2 and hormonal receptors cooperate in the resistance to targeted therapy in breast cancer; data from BOLERO-2 and BOLERO-3 trials suggest that targeting PI3K pathway can reverse the resistance to HER2 and endocrine therapy in pre-treated patients. A better characterization of mechanisms of resistance should be done in order to identify patients that will benefit of targeted therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor San-Gang Wu (Department of Radiation Oncology, Xiamen Cancer Center, the First affiliated Hospital of Xiamen University, Xiamen, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.42). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer, 2013. Accessed on 11/Aug/2016. Available online: http://globocan.iarc.fr,
- Loibl S, Majewski I, Guarneri V, et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol 2016;27:1519-25. [Crossref] [PubMed]
- Tokunaga E, Kimura Y, Mashino K, et al. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer 2006;13:137-44. [Crossref] [PubMed]
- Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007;12:395-402. [Crossref] [PubMed]
- The TCGA publication guidelines. Available online: Cbioportal.org
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61-70. [Crossref] [PubMed]
- Castaneda CA, Lopez-Ilasaca M, Pinto JA, et al. PIK3CA mutations in Peruvian patients with HER2-amplified and triple negative non-metastatic breast cancers. Hematol Oncol Stem Cell Ther 2014;7:142-8. [Crossref] [PubMed]
- Matosin N, Fernandez-Enright F, Lum JS, et al. Molecular evidence of synaptic pathology in the CA1 region in schizophrenia. NPJ Schizophr 2016;2:16022. [Crossref] [PubMed]
- Hanker AB, Pfefferle AD, Balko JM, et al. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proc Natl Acad Sci U S A 2013;110:14372-7. [Crossref] [PubMed]
- Majewski IJ, Nuciforo P, Mittempergher L, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol 2015;33:1334-9. [Crossref] [PubMed]
- Pereira B, Chin SF, Rueda OM, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun 2016;7:11479. [Crossref] [PubMed]
- Bosch A, Li Z, Bergamaschi A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med 2015;7:283ra51 [Crossref] [PubMed]
- Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 2014;32:3883-91. [Crossref] [PubMed]
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796-804. [Crossref] [PubMed]
- de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol 2014;15:1137-46. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- von Minckwitz G. A step towards a HER2-positive breast cancer super family. Lancet Oncol 2015;16:745-6. [Crossref] [PubMed]
- Gómez HL, Castañeda CA, Vigil CE, et al. Prognostic effect of hormone receptor status in early HER2 positive breast cancer patients. Hematol Oncol Stem Cell Ther 2010;3:109-15. [Crossref] [PubMed]
- Cheng H, Liu P, Ohlson C, et al. PIK3CA(H1047R)- and Her2-initiated mammary tumors escape PI3K dependency by compensatory activation of MEK-ERK signaling. Oncogene 2016;35:2961-70. [Crossref] [PubMed]
- Hurvitz SA, Andre F, Jiang Z, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol 2015;16:816-29. [Crossref] [PubMed]
- André F, O'Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014;15:580-91. [Crossref] [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [Crossref] [PubMed]
- Le X, Antony R, Razavi P, et al. Systematic Functional Characterization of Resistance to PI3K Inhibition in Breast Cancer. Cancer Discov 2016;6:1134-1147. [Crossref] [PubMed]


