
The accuracy and validity of HPV testing through self-collection with tampons for cervical cancer screening
Introduction
Cervical cancer is a highly preventable disease with regular screening and detection and removal of precancerous lesions. The Pap test was developed in the 1920’s for detection of precancerous and cancerous cells of the cervix, but did not begin becoming widely used as the first cancer screening test until the 1960’s. Prior to widespread use of the Pap test, cervical cancer was the leading cancer cause of death of women in the U.S; cervical cancer is now reduced to not even being in the top ten (1).
The Pap test is known to have a high specificity for detection of high-grade cervical intraepithelial neoplasia and cancer at around 96%, but a much lower sensitivity at around 50%, potentially leaving many lesions undetected. However, a comparison of multiple studies in North American and Europe have demonstrated the high variability in sensitivity, with lower sensitivity increasing the proportion of cancers occurring in women assumed to be adequately screened (2).
The HPV DNA test looks for oncogenic types of human papillomavirus (HPV) that cause almost all cases of cervical cancer. The HPV test has been shown to have better efficacy to find early and advanced cervical cancers as compared to Pap testing in several studies (3,4). In 2012, HPV testing was incorporated in addition to the Pap test in the new screening guidelines for the prevention and early detection of cervical cancer in the United States. Among women at average risk (no HIV infection, organ transplant, DES exposure, etc.), the American Cancer Society recommends screening with Pap test alone every 3 years for women 21 to 29 years of age, and the preferred screening method for women 30 to 65 is HPV and Pap “co-testing” every 5 years (5).
Studies have demonstrated that HPV testing is slightly less specific than Pap testing (90.75), but its high reproducibly and ease of monitoring reduces the variability in its high sensitivity (96.1%) in detecting CIN2+. This high sensitivity suggests that the HPV test could be used as the primary test with Pap testing reserved as follow-up for those who are positive (2).
In 2014, the Food and Drug Administration (FDA) approved the use of one HPV DNA test (Cobas HPV test, Roche Molecular Systems, Inc.) as a first-line primary screening test for use alone for women age 25 and older (6). In the study that led to this approval, Pap and HPV testing were undertaken on 42,209 eligible women, and women with a positive finding in either test were referred for colposcopic evaluation. Results showed HPV primary screening in women ≥25 years was as effective as a hybrid screening strategy that uses Pap testing if 25–29 years and co-testing if ≥30 years (7). HPV testing alone could replace Pap testing for cervical cancer screening among women ≥25 years, reducing the overall numbers of tests and cost of testing.
However, there are still many potential barriers to adherence to cervical cancer screening under current guidelines or using HPV DNA detection alone. Both of these methods require a clinic visit. While adherence to cervical cancer screening guidelines are generally high in the U.S. (83%), this percentage decreases for lower-income women (8). Further, a recent study indicates that the majority of women in the U.S. 45–64 years of age do not obtain care from a gynecologist which could further decrease adherence to screening guidelines that would require a clinic visit (9). Reasons such as lost time from work, lack of transportation or childcare, lack of health care coverage, lack of knowledge, or embarrassment are barriers to clinic-based testing and therefore adherence to cervical cancer screening guidelines (10-12). Self-sampling at home may be an ideal way to overcome the above barriers to cervical cancer screening, and several studies have shown self-sampling to be preferred by more women which could increase the screening rate (13-15).
In this prospective randomized study, accuracy and validity of HPV testing with a sample self-collected with a tampon were observed. To our knowledge, this is the first study to explore the performance of HPV testing with self-collecting at home with a tampon in randomized controlled trial. Home testing via methods such as this can assist with overcoming barriers seen in clinic-based testing.
Methods
Participants and recruitment
A total of 120 subjects were randomly assigned to the Self-collection Arm or Clinic Arm equally, from October 2012 to May 2015. The participants were recruited from three medically underserved neighborhoods in New Orleans (Treme, Central City, and Hollygrove) and from the Louisiana Breast and Cervical Cancer Health Program (LBCHP), which provides screening for low-income, uninsured women. Eligible participants were women living in the identified areas or who were previous participants in LBCHP, who were 21 years of age and over, had not had a hysterectomy, were English speaking, and were at least 1 year past their last Pap test. At the time this study was proposed, annual screening was still considered appropriate and given the self-report of last Pap test and lack of medical history on participants, was continued throughout the study. Potential participants who had a history of gynecologic cancer or toxic shock syndrome, or were currently pregnant were not eligible. The study was approved by the LSU Health-New Orleans institutional review board and informed consent was obtained from all participants.
Procedures in the Self-collection Arm
Participants in the Self-collection Arm were given a kit which contained two tampons, a small pouch of personal lubricant, one plastic tube, one absorbent paper, one piece of bubble wrap, one biologics bag, an instruction sheet, a patient identification card, and a pre-addressed stamped postal service approved mailer, and instructions were reviewed with the participant by the Outreach Worker. Instructions for the self-collecting were as follows: the participants should abstain from vaginal intercourse for 24 h prior to performing the test; participants should not complete the testing if they were experiencing their menses, but postpone testing until 3 days after menses have ceased; tampon should be inserted and left in place for 2 h. After removing by only touching the string, the tampon should be placed in the plastic tube which is then wrapped in the absorbent paper and bubble wrap and placed in the biologics bag following postal regulations. The participant was instructed to complete the identification card, which was prepopulated with the study identification number, to indicate date of collection. The participant was instructed to place the bag and the patient identification card in the pre-stamped and addressed fiberboard mailer and to mail back to the investigators within 24 h of removing the tampon. Participants who did not return the tampon were contacted up to two times to remind before being categorized as non-adherent.
To process the tampons, the tampon was placed in the barrel of 60 mL syringe, 10 mL of PreservCyt (Cytyc) were added to the tampon, and the fluid extracted by passing through the syringe. The fluid was then tested for HPV types 16 and 18 and high-risk HPV (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) using the Roche Cobas system using similar mechanisms as for routine reflex Pap testing at the pathology laboratory of the Interim LSU Hospital. Standard laboratory practices were used to insure accuracy of measures.
After the sample was received, participants in this arm were contacted to complete a satisfaction survey and to arrange a clinic visit for a Pap test, HPV test, and pelvic exam in the gynecology clinic. Participants were offered $25 gas cards to assist with transportation. Participants with abnormal results on any of the clinic tests were contacted to arrange for follow-up testing and subsequent treatment if needed.
Procedures in the Clinic Arm
Participants in the Clinic Arm were assisted by Community Health Workers in obtaining an appointment for a Pap test, HPV test, and pelvic exam in the gynecology clinic. Participants were offered $25 gas cards to assist with transportation. After completion of clinic testing, the participants were administered a brief satisfaction survey. Participants who did not attend the scheduled clinic visit were contacted up to two times to reschedule before being categorized as non-adherent. Participants with abnormal results on any of the clinic tests were contacted to arrange for follow-up testing and treatment if needed.
Statistical analysis
Z-test was used to compare compliance as well as detection rate in the two arms. Sensitivity and specificity of self-collected samples were calculated with HPV testing from clinic samples as the reference test. Logistic regression was conducted to analyze the association between test validity and age, tampon use history, days between sample collection and sample receipt (ColtoRec), and days between sample receipt and sample test (RectoTest). Fisher’s exact test was employed for comparing the ColtoRec between the valid and invalid sample groups. Analyses were performed using SAS 9.4.
Results
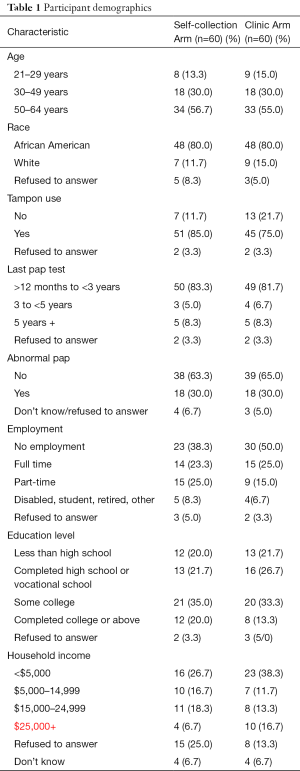
From October 2012 to May 2015, 120 subjects were randomly assigned to the Self-collection Arm or Clinic Arm, 60 each. As shown in Table 1, overall participants were primarily African American (82.8%), unemployed or employed only part-time (64.2%), and with incomes less than $25,000 (62.5%). The two arms were comparable in age, tampon use history, last Pap test, abnormal Pap history, ethnicity, employment, education level, and household income.
Full table
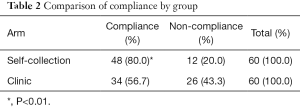
The analysis of test compliance was performed and results are shown in Table 2. Forty-eight (80.0%) out of 60 subjects in the Self-collection Arm returned tampons in the proper packaging with identification card, while only 34 (56.7%) out of 60 subjects in the Clinic Arm presented at the clinic for testing. Z-test showed that self-collection compliance is significantly higher than clinic compliance (P<0.01).
Full table
Detection rate with self-collection samples
In this study, 35 out of 48 subjects who returned tampons were positive for beta-globin (detection of human DNA, an indication that the tampon contacted human tissue). Thirteen tests from the self-collected samples were reported as “invalid” by the lab for lack of beta-globin and were therefore inadequate samples. Based on the beta-globin test, the rate of adequate samples with self-collecting was 72.9% (35/48). The total adequate sample rate for all participants in the Self-collection Arm was 58.3% (35/60), which was no different from the number of adequate samples in the Clinic Arm (56.7%, 34/60) (Table 3). Therefore, although there was a significantly increased compliance for self-collection compared to clinic testing, when the ability to detect DNA on the received samples is considered, the adequate sample rate is not different.

Full table
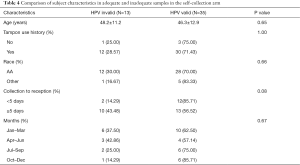
The association of an invalid result from self-collected samples with other factors was analyzed. As shown in Table 4, age, race, tampon use history, and the season in which the sample was collected were not related to an adequate sample being collected. However, when the samples were stratified by the number of days from the date of collection indicated on the identification card to the date received by the lab (<5 days—short interval, >5 days—long interval), the adequate sample rate in the short interval group was 85.71% versus 56.52% in the long interval group. This difference approaches significance using Fishers exact test (P=0.08).
Full table
When the number of days from the date of collection as indicated on the identification card to receipt of test was further analyzed with logistic regression, it showed that the inadequate sample result was close to being significantly associated with the long interval (P=0.08) based on a significance level of 0.05. The odds of having an inadequate sample in the long interval group was 4.62 times of the odds in the short interval group, odds ratio 4.62 (95% CI, 0.84–25.49).
Sensitivity and specificity of HPV test with self-collected samples
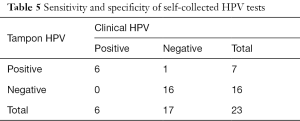
In our study, of the 48 women returning the self-collected sample, 34 attended the clinic visit. Only one participant that did not return the self-collected sample attended the clinic visit. Of the 35 women returning tampons that had valid HPV results, 23 participants completed the clinic visit as well and therefore had paired HPV results from both self-collected and clinic samples. As shown is Table 5, with the HPV result from clinic sample as the reference test, the sensitivity of HPV in self-collected sampling was 100% (6/6), specificity was 94.1% (16/17), positive predictive value was 85.7% (6/7), and negative predictive value is 100% (16/16). Kappa test shows there is a strong agreement in test results between self-collected and clinical sample (Kappa=0.89 with 95% CI, 0.69–1.00).
Full table
Conclusions
Self-collection for cervical cancer screening has been shown to be highly acceptable among women (16). The procedure for self-collection is very easy: a kit for self-collection and return to a testing laboratory. Women may prefer this test because it is less painful, less embarrassing, and less stressful (17). Self-collection especially benefits those who are at high-risk of cervical cancer and non-adherent to regular office-based screening (18).
Self-collected samples in our study had very good sensitivity and specificity, when compared to a clinic sample. While the compliance with returning the self-collected samples was significantly higher than compliance with attending clinic visits, the percentage of inadequate samples was 27% in the Self-collection Arm and 0% in the Clinic Arm. This resulted in an overall adequate sample rate that was not different between the two arms (58.3% vs. 56.7%). We analyzed the possible reasons for the inadequate HPV tests in self-collected samples. Age or tampon use history were not related. The relationship between adequate samples and less than 5 days between sample collection and receipt of sample by the lab approached significance (P=0.08). Analysis of the time of year that the sample was mailed revealed no association between average temperatures and inadequate samples. If exposure to heat had been a factor in DNA degradation, one would have expected more inadequate samples in the summer months, which was not the case.
While the exact reason for the association between length of time from collection to receipt of specimen is not clear, only 37 of the 48 self-collected samples received had indicated collection date on the ID card. A larger sample size could help answer this question. The negative beta globin tests would support the hypothesis that the tampons were not inserted at all or for very long, but how this would be related to the longer interval between self-collection and receipt by lab is unclear.
While the study is limited by a relatively small sample size, it showed that self-collected samples had similar test results as clinic samples, and the accuracy of the result from self-collected samples was very good. Around 27% of self-collected samples were inadequate for testing, which was shown to possibly be related to longer intervals between the time reported for sample collection and the time the sample was received by the lab. The reason for this association is unclear, but could be related to a failure to follow instructions.
Home-sampling for HPV has potential to overcome many of the barriers to clinic testing in high risk women who are unable to adhere to screening recommendations. This approach would also allow health care providers to focus limited resources on the hard to reach women with positive test results rather than on the entire population. The tampon is an inexpensive method and is widely recognized by women. However, further study is needed to determine the reason for the high number of inadequate samples with self-collection.
Acknowledgments
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R21CA157263. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tung-Sung Tseng, Dung-Tsa Chen, Hui-Yi Lin) for the series “Social Behavioral and Genetic Risk factors for Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.54). The series “Social Behavioral and Genetic Risk factors for Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the LSU Health-New Orleans institutional review board and informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts & Figures, 2016. Atlanta: American Cancer Society, 2016.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006;119:1095-101. [Crossref] [PubMed]
- Agorastos T, Chatzistamatiou K, Katsamagkas T, et al. Primary screening for cervical cancer based on high-risk human papillomavirus (HPV) detection and HPV 16 and HPV 18 genotyping, in comparison to cytology. PLoS One 2015;10:e0119755 [Crossref] [PubMed]
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009;360:1385-94. [Crossref] [PubMed]
- American Cancer Society. Cervical Cancer Prevention and Early Detection. 2014. Retrieved July 2015. Available online: http://www.cancer.org/acs/groups/cid/documents/webcontent/003167-pdf.pdf
- FDA approves first human papillomarvirus test for primary cervical cancer screening. 4/24/2014. Available online: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015;136:189-97. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services; 2014.
- Raffoul MC, Petterson SM, Rayburn WF, et al. Office Visits for Women Aged 45-64 Years According to Physician Specialties. J Womens Health (Larchmt) 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Behbakht K, Lynch A, Teal S, et al. Social and cultural barriers to Papanicolaou test screening in an urban population. Obstet Gynecol 2004;104:1355-61. [Crossref] [PubMed]
- Oscarsson MG, Wijma BE, Benzein EG. 'I do not need to... I do not want to... I do not give it priority...'--why women choose not to attend cervical cancer screening. Health Expect 2008;11:26-34. [Crossref] [PubMed]
- Östensson E, Alder S, Elfström KM, et al. Barriers to and facilitators of compliance with clinic-based cervical cancer screening: population-based cohort study of women aged 23-60 years. PLoS One 2015;10:e0128270 [Crossref] [PubMed]
- Rosenbaum AJ, Gage JC, Alfaro KM, et al. Acceptability of self-collected versus provider-collected sampling for HPV DNA testing among women in rural El Salvador. Int J Gynaecol Obstet 2014;126:156-60. [Crossref] [PubMed]
- Duke P, Godwin M, Ratnam S, et al. Effect of vaginal self-sampling on cervical cancer screening rates: a community-based study in Newfoundland. BMC Womens Health 2015;15:47. [Crossref] [PubMed]
- Crosby RA, Hagensee ME, Vanderpool R, et al. Community-Based Screening for Cervical Cancer: A Feasibility Study of Rural Appalachian Women. Sex Transm Dis 2015;42:607-11. [Crossref] [PubMed]
- Giorgi Rossi P, Fortunato C, Barbarino P, et al. Self-sampling to increase participation in cervical cancer screening: an RCT comparing home mailing, distribution in pharmacies, and recall letter. Br J Cancer 2015;112:667-75. [Crossref] [PubMed]
- Hanley SJ, Fujita H, Yokoyama S, et al. HPV self-sampling in Japanese women: A feasibility study in a population with limited experience of tampon use. J Med Screen 2016;23:164-70. [Crossref] [PubMed]
- Racey CS, Withrow DR, Gesink D. Self-collected HPV testing improves participation in cervical cancer screening: a systematic review and meta-analysis. Can J Public Health 2013;104:e159-66. [PubMed]


