
Is there a role for epidermal growth factor receptor inhibition in the treatment of advanced esophagogastric tumours?
Esophageal cancers, including esophagogastric junction tumours are the sixth leading cause of cancer mortality worldwide (1). While the incidence of adenocarcinomas predominate in developed countries (2), squamous-cell carcinomas of the esophagus demonstrate an association with lower socioeconomic status (3). Gastric cancers, the vast majority are adenocarcinomas, accounting for 700,000 deaths per year, rendering this the second commonest cause of cancer mortality worldwide (4). In recent years, the site of tumor has shifted from predominantly occurring in the distal stomach to now at, or near the gastroesophageal junction (GEJ) (5). This has been attributed to a change in the prevalence of underlying risk factors for developing gastric cancer, with a decline in chronic Helicobacter pylori infections and an increase in gastroesophageal reflux and obesity (5). For both esophageal and gastric cancer, chemotherapy is of proven benefit in palliating symptoms with a modest improvement in survival, however durable responses are infrequent. The therapeutic benefits of combination chemotherapy regimens must be balanced against potential toxicity in a patient population where nutritional and general debility issues frequently coexist (2).
The anti-epidermal growth factor receptor monoclonal antibodies (EGFR mAbs), cetuximab and panitumumab, competitively inhibit the epidermal growth factor receptor (EGFR) extracellular domain (6). They are widely used in the treatment of colorectal cancer, head and neck and other tumour types; improving response, progression-free and overall survival when used in combination with chemotherapy or as single agents. The rationale for evaluating the use of EGFR-inhibitors (EGFR-I) in esophagogastric cancer is based on the overexpression of EGFR in 64% of esophageal adenocarcinomas (7), 71–83% of squamous cancers (8,9) and just under 50% of gastric cancers (10), with a clinicopathological evaluation of 78 cases of gastric tumours from a single institution demonstrating a higher likelihood of EGFR expression in poorly differentiated tumours of a more advanced stage (11). EGFR gene amplification has been reported in 8% of esophageal adenocarcinomas (12,13).
Preclinical studies have demonstrated constitutive activation of the EGFR signaling pathway in esophagogastric cancer cell lines (14). Inhibition of the receptor with EGFR mAbs reduces signaling in the EGFR pathway through reduced phosphorylation of EGFR and AKT (15). In vitro, the addition of anti-EFGR antibodies to chemotherapy demonstrated synergistic inhibition of cell proliferation and enhanced apoptosis (13,15-17). In colorectal cancer patients, the use of EGFR-I are restricted to the 40% of patients wildtype for the RAS gene, with multiple studies showing lack of benefit in RAS mutated tumours. However, it is rare (<10%) to see KRAS mutations in esophagogastric cancer (18-20); this low prevalence preventing its utility as a predictive biomarker.
Here we provide a perspective on the recently published randomised phase II CALGB 80403 (Alliance)/E1206 study comparing cetuximab efficacy and toxicity when combined with three different standard chemotherapy backbones in patients with metastatic esophagogastric cancer and discuss its clinical practice implications. This study commenced at a time when there was great hope that the addition of biological agents to standard chemotherapy would improve outcomes in a disease where little progress had been made for some time. The trial sought to identify the optimum chemotherapy—EGFR-I combination using standard chemotherapies active in metastatic esophagogastric cancer (1), including oxaliplatin (1), docetaxel (21), capecitabine (22) and irinotecan (18,21,23-25), to give rise to the optimal regimen to be taken forward into randomised phase III trials. The rationale for this direct comparison was further stimulated by emerging studies in colorectal cancer (CRC) suggesting that the chemotherapy partner for anti-EGFR treatments may influence efficacy, although this still remains controversial (26-30). Unfortunately, large studies published during the trial period showed no benefit for the addition of EGFR-I in this disease (13,31), so that the conclusion of 80,403 demonstrating no statistical difference between the regimens of little clinical importance (32).
Unlike CRC, no biomarker selection for EFGR-I use in esophagogastric cancer has been identified. Earlier phase I/II studies contained an unselected population, with the exception of the phase II study by Lorenzen et al. which only included patients with evidence of EGFR overexpression by standardised immunohistolochemical (IHC) testing (19). However, IHC overexpression has not been shown to be a predictive marker in any tumour subtype (33-35). We reflect on these earlier studies to provide context for our discussion on the recent CALGB 80403 (Alliance)/ E1206 study publication.
Phase I/II studies in first line treatment of metastatic esophagogastric cancer
Two early phase I/II studies were conducted in patients with gastric cancer and GEJ tumours comprising approximately half of each study population (6,20). The 2010 Lordick study was a single arm study of 52 participants evaluating response rate (RR), safety and efficacy of cetuximab addition to oxaliplatin and 5-fluorouracil (FU) (6). An encouraging RR of 65% was seen, with a suggestion of higher response in GEJ tumours compared to a gastric primary (77% vs. 54%). The median time to progression (TTP) was 7.6 months with a 9.5-month median overall survival (OS). There was no clear association with degree of EGFR expression by IHC.
The Pinto et al. study was a single arm trial in 72 patients of cetuximab addition to cisplatin and docetaxel, also in a predominant gastric cancer population (82%) (20). RR was encouraging at 41% with a median duration of response of 5 months and median OS of 9 months. This RR was higher than reported in historical docetaxel plus cisplatin trials (36,37). This study demonstrated a non-significant trend between increased severity of skin toxicity and treatment activity, an observation seen in other tumour types (38,39). A small number (n=16) of patients who had at least stable disease (SD) after 6 cycles on trial went on to maintenance treatment with cetuximab alone, demonstrating a trend for both longer TTP, 9.2 vs. 6.6 months (P=0.10) and improved OS, 19.8 vs. 7.7 months (P=0.22). Overall, treatment was reasonably tolerated and chemotherapy toxicities were not significantly increased by addition of cetuximab. The lower rates of grade 3–4 toxicities and febrile neutropenia compared to that reported in the SAKK42/99 trial (36) can be explained by the lower doses of both chemotherapy agents (20).
A multicentre phase II Austrian study evaluated the safety and feasibility of a unique chemotherapy backbone of oxaliplatin and irinotecan with cetuximab in 51 patients with advanced gastric cancer (40). The triplet was deemed safe (primary endpoint); secondary endpoints of efficacy were evaluable in 35 patients. One patient (3%) demonstrated a CR, 21 patients (60%) a PR and 7 patients (20%) SD. The median TTP was 25 weeks, with median OS of 38 weeks.
Another encouraging study reported by Moehler et al. reporting a 48% RR in 48 patients with the addition of cetuximab to irinotecan plus 5-FU with promising longer term outcomes [median progression free survival (PFS) 9 months and median OS 16.5 months] (18). Predictive biomarkers were evaluated in 34 patients; KRAS, BRAF and PIK3CA mutations were detected in 3 (9%), 4 (12%) and 2 (6%) of tumours respectively, each mutually exclusive. Amongst the tumours with KRAS mutations, two exhibited a response to treatment: one complete response (CR) and one partial response (PR). Whilst no patients whose tumours harboured BRAF or PIK3CA mutations responded, the low numbers limit conclusions. EGFR expression by IHC was detected in 26/39 (67%) of tumours, with no correlation to outcome. There was no statistical difference in best tumour response or survival based on development of a treatment-related rash.
Not all early phase studies reported high RRs however, with a German study by Lorenzen et al. reporting modest RRs (<40%) with no differences in PFS or OS in a 2-arm randomised design of cisplatin/5-fluorouracil (CF) ± cetuximab (19). Some data of interest arose from the unplanned subgroup analysis of the small number of patients in the chemotherapy arm who crossed over to receive cetuximab as monotherapy (n=2) or had cetuximab added to continued CF chemotherapy (n=3) on progression. One of the patients who received cetuximab monotherapy achieved a PR and the other achieved SD; one of the three patients where cetuximab was added to refractory chemotherapy achieved a PR. The median PFS from time of crossover was 6.8 months and median OS 7.1 months in this small cohort. This raised speculation also about whether RECIST measured RR was the most appropriate method of characterising cetuximab benefit, or whether cetuximab could reverse chemotherapy refractoriness, as was being discussed in other tumour types.
Similarly, a 150-patient phase II study evaluated a docetaxel and oxaliplatin backbone ± cetuximab (41). There was a near even split of patients with gastric and GEJ tumours. The primary endpoint of PFS was not improved and there was a greater rate of treatment discontinuation due to adverse events (AEs) with the addition of cetuximab. The RR improved from 27% to 38% with its addition.
Rather than cetuximab, the ATTAX3 trial evaluated the safety and efficacy of adding panitumumab to chemotherapy in patients with advanced esophagogastric cancer (42). Of the 71 participants, 61% had esophageal or GEJ tumours and 90% were adenocarcinoma. No benefit was seen in RR, PFS or OS, but increased toxicity.
Phase I/II studies in refractory metastatic and locally advanced disease
A 35-patient phase II U.S. study evaluated the safety and efficacy of single agent cetuximab beyond the first line setting (43). This included one third with advanced esophageal tumours, 23% with GEJ tumours and the remainder gastric. Despite rash (all grades) being reported in 77% of patients, the primary endpoint of RR was documented at only 3%. The median PFS and OS were 1.6 months and 3.1 months respectively.
Two single arm, phase II studies evaluating the addition of cetuximab to 5-fluorouracil and oxaliplatin (FOLFOX) (44), or irinotecan and cisplatin (IC) chemotherapy (45) with radiation, demonstrated mixed results in the locally advanced esophageal cancer setting. The 79-patient trial using FOLFOX plus cetuximab met its primary endpoint with an objective response rate (ORR) of 77% (expected rate >50%), with 40% of patients achieving a CR (44); the study evaluating the IC backbone suffered from poor accrual, demonstrating significant toxicity in the 19 evaluable patients and an insufficient pCR rate to warrant further evaluation (45).
SCOPE 1 was another negative phase II study evaluating cetuximab addition to chemoradiation with cisplatin and capecitabine (46), this time as definitive therapy. The primary endpoint was freedom from treatment failure at 24 weeks. Recruitment ceased due to meeting criteria for futility before the planned continuation to a phase III study, with boundaries set based on treatment-failure-free rate of <60% at week 24 not deemed sufficient to warrant further investigation, but a rate of >75% worthy of further study. All study participants (n=258) had esophageal cancer with the majority having squamous histology (~75%). Fewer patients were free from treatment failure at 24 weeks in the cetuximab pus chemoradiation arm (66.4%) compared to chemoradiation alone. Median OS was also shorter in the former, with an adjusted hazard ratio (HR) of 1.53 (95% CI, 1.03–2.27). The study concluded that the addition of cetuximab to a standard definitive chemoradiation regimen resulted in increased toxicity and postulated that the reduced efficacy resulted from the reduction in delivery of both components of standard chemoradiation.
Phase III trials
The disappointment of existing treatments together with promising clinical activity in many (but not all) phase II cetuximab-chemotherapy combination trials led to an evaluation of cetuximab in larger phase III trials. This coincided with excitement regarding the efficacy of the EGFR-I in metastatic CRC.
The EXPAND phase III study evaluated cetuximab benefit when added to capecitabine and cisplatin in patients with previously untreated, advanced gastric cancer (31). There was no molecular selection or stratification. The first patient was enrolled in June 2008 and last in December 2010, with the study published in 2013. Only 17% of patients had GEJ tumours. The primary endpoint, median PFS, for the 455 participants who received cetuximab + chemotherapy was 4.4 vs. 5.6 months for the 449 patients receiving chemotherapy alone (HR 1.09; 95% CI, 0.92–1.29; P=0.32). The regimen was toxic with 83% and 77% of participants in the cetuximab + chemotherapy and chemotherapy alone arms experiencing grade 3–4 AEs, respectively. This study made the disappointing conclusion that there was no benefit from the addition of cetuximab to a capecitabine-cisplatin chemotherapy backbone.
REAL3 was a phase II/III study that evaluated the safety and efficacy of adding panitumumab to epirubicin, oxaliplatin and capecitabine (EOC) in an unselected patient population with advanced esophagogastric adenocarcinoma. The phase II component, which recruited patients in 2008−2009, identified a high level of toxicity that mandated dose modifications (47) before proceeding to the phase III component (13). Enrolment was completed in October 2011. Despite great anticipation, this study which was published in 2013 did not demonstrate any benefit for the addition of panitumumab; on the contrary there was evidence of harm with the OS HR 1.37 (95% CI, 1.07–1.76; P=0.013). Subgroup analyses revealed no clear biomarkers predictive for panitumumab benefit, although the presence of a KRAS mutation in 10 participants showed a nonsignificant improvement in OS (HR 0.23; 95% CI, 0.05–1.15). In contrast to data in CRC, OS was reduced amongst participants with KRAS wild type tumours who received panitumumab (n=164), HR 1.50 (95% CI, 1.03–2.18). The dose intensity did not vary significantly between study arms, thus was not an explanation for the inferior outcome. As expected, there was increased toxicity with the addition of the EGFR-I (13). These studies are summarised in Table 1.
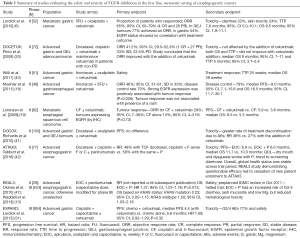
Full table
In a combined modality setting, a phase III trial evaluated the addition of cetuximab to a paclitaxel and cisplatin backbone with radiation in 344 patients with locally advanced esophageal cancer (T1N1M0 or T2-4 any N, M0 or any T/N, M1a) treated without surgery. This study demonstrated no improvement in its primary endpoint of OS, nor in RR, irrespective of histological subtype (48).
The recent publication of the randomised phase II Cancer and Leukemia Group B (CALBG) 80403 (Alliance)/E1206 study reported in July 2016 by Enzinger et al. in the Journal of Clinical Oncology, is now discussed having set the scene of its historical context. This study aimed to compare differences in the ORR (the primary endpoint) with cetuximab when used in combination with three different chemotherapy backbones—epirubicin, cisplatin and 5-fluorouracil (ECF), 5-FOLFOX or IC (32). Planned secondary objectives included OS, PFS and time to treatment failure (TTF). This multicentre trial was a collaborative effort by CALBG and ECOG, recruiting a total of 245 patients with metastatic esophageal (56%) and GEJ tumours (43%) across the US, in a time period prior to reporting of the large phase III trials discussed above. Ninety-one percent of patients had adenocarcinoma and there was no molecular selection. Whilst the data from this trial was maturing following full enrolment, results of the large REAL-3 and EXPAND studies were published. This prompted unplanned pair-wise comparisons of TTF, PFS, OS and toxicity end points across the three regimens. For the ECF, FOLFOX and IC regimens respectively, RR was 60.9%, 54.3% and 45.0%; median OS was 11.6, 11.8 and 8.6 months; and median PFS 7.1, 6.8 and 4.9 months. Participants who received FOLFOX plus cetuximab required less treatment modifications (P=0.013) and withdrawal from study, due to AEs compared to the other regimens. The study authors concluded that there was a similar efficacy when adding cetuximab to ECF as to FOLFOX, with the latter reported as the better tolerated regimen by toxicity grading. Cetuximab addition to IC seemed to be the least effective and most toxic regimen, although differences were not statistically significant. There was no formal evaluation of quality of life (QoL). Tissue collection was not reported nor any translational work proposed within this study (32).
The CALGB 80403 (Alliance)/E1206 study, in the context of large negative phase III studies of adding EGFR-I to chemotherapy for advanced esophagogastric cancer, does not yield conclusions that would reinvigorate this direction of therapeutic manipulation. This data can be used to support the FOLFOX regimen as a standard of care for patients with advanced esophageal or GOJ cancer, although it was not superior to the other two regimens.
Sadly, once again, we see a new therapeutic class failing when combined with chemotherapy in large phase III trials despite (some) encouraging phase II trials. This underscores the importance of robust large trials but additionally reinforces the need for translational research and QoL data collection. It also highlights the enormous effort and lengthy process of bringing a trial from inception to fruition. The “retrospectoscope” is indeed a powerful tool. The CALGB’s methodical evaluation of testing the optimal chemotherapy doublet in the phase II setting should be applauded and research efforts to identify molecular predictive factors must continue, as it is possible that there is a subgroup of patients with esophageal and gastric cancer that may indeed benefit from targeting the EGFR pathway.
The historical publication bias against negative trials has been well recognised, hence the prominent publication and editorial and review articles regarding this trial allow for appropriate reflection despite the outcome cementing, rather than changing current clinical practice. We can conclusively state that there is no role for the addition of EGFR-inhibitors to chemotherapy in unselected patients with advanced esophagogastric cancer, but that we can only come to such conclusions with the conduct of high quality clinical trials made possible because of the dedication of patients and their families.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Lei Huang, MD, MSc, MBBS, PhD candidate (Department of Gastrointestinal Surgery, the First Affiliated Hospital of Anhui Medical University, Hefei, China; German Cancer Research Center (DKFZ), Heidelberg, Germany).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.29). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Brown LM, Hoover R, Silverman D, et al. Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. Am J Epidemiol 2001;153:114-22. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Sibertin-Blanc C, Ciccolini J, Norguet E, et al. Monoclonal antibodies for treating gastric cancer: promises and pitfalls. Expert Opin Biol Ther 2016;16:759-69. [Crossref] [PubMed]
- Lordick F, Luber B, Lorenzen S, et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer 2010;102:500-5. [Crossref] [PubMed]
- Itakura Y, Sasano H, Shiga C, et al. Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer 1994;74:795-804. [Crossref] [PubMed]
- Torzewski M, Sarbia M, Verreet P, et al. The prognostic significance of epidermal growth factor receptor expression in squamous cell carcinomas of the oesophagus. Anticancer Res 1997;17:3915-9. [PubMed]
- Yacoub L, Goldman H, Odze RD. Transforming growth factor-alpha, epidermal growth factor receptor, and MiB-1 expression in Barrett's-associated neoplasia: correlation with prognosis. Mod Pathol 1997;10:105-12. [PubMed]
- Atmaca A, Werner D, Pauligk C, et al. The prognostic impact of epidermal growth factor receptor in patients with metastatic gastric cancer. BMC Cancer 2012;12:524. [Crossref] [PubMed]
- Gao M, Liang XJ, Zhang ZS, et al. Relationship between expression of EGFR in gastric cancer tissue and clinicopathological features. Asian Pac J Trop Med 2013;6:260-4. [Crossref] [PubMed]
- Miller CT, Moy JR, Lin L, et al. Gene amplification in esophageal adenocarcinomas and Barrett's with high-grade dysplasia. Clin Cancer Res 2003;9:4819-25. [PubMed]
- Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin and capecitabine with or without panitumumab for patients with previously untreated advanced esophagogastric cancer (REAL3): a randomized, open-label phase 3 trial. Lancet Oncol 2013;14:481-89. [Crossref] [PubMed]
- Di Fiore PP, Pierce JH, Fleming TP, et al. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell 1987;51:1063-70. [Crossref] [PubMed]
- Fukuda K, Saikawa Y, Takahashi M, et al. Antitumor effect of cetuximab in combination with S-1 in EGFR-amplified gastric cancer cells. Int J Oncol 2012;40:975-82. [PubMed]
- Luo HY, Wei W, Shi YX, et al. Cetuximab enhances the effect of oxaliplatin on hypoxic gastric cancer cell lines. Oncol Rep 2010;23:1735-45. [PubMed]
- Kobunai T, Watanabe T, Fukusato T. Antitumour activity of S-1 in combination with cetuximab on human gastric cancer cell lines in vivo. Anticancer Res 2011;31:3691-6. [PubMed]
- Moehler M, Mueller A, Trarbach T, et al. Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol 2011;22:1358-66. [Crossref] [PubMed]
- Lorenzen S, Schuster T, Porschen R, et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2009;20:1667-73. [Crossref] [PubMed]
- Pinto C, Di Fabio F, Barone C, et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study). Br J Cancer 2009;101:1261-8. [Crossref] [PubMed]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. [Crossref] [PubMed]
- Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009;20:666-73. [Crossref] [PubMed]
- Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 2008;19:1450-7. [Crossref] [PubMed]
- Moehler M, Kanzler S, Geissler M, et al. A randomized multicenter phase II study comparing capecitabine with irinotecan or cisplatin in metastatic adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 2010;21:71-7. [Crossref] [PubMed]
- Curran D, Pozzo C, Zaluski J, et al. Quality of life of palliative chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction treated with irinotecan combined with 5-fluorouracil and folinic acid: results of a randomised phase III trial. Qual Life Res 2009;18:853-61. [Crossref] [PubMed]
- Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011;22:1535-46. [Crossref] [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346-55. [Crossref] [PubMed]
- Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012;30:1755-62. [Crossref] [PubMed]
- Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103-14. [Crossref] [PubMed]
- Chan DL, Pavlakis N, Shapiro J, et al. Does the Chemotherapy Backbone Impact on the Efficacy of Targeted Agents in Metastatic Colorectal Cancer? A Systematic Review and Meta-Analysis of the Literature. PLoS One 2015;10:e0135599 [Crossref] [PubMed]
- Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490-9. [Crossref] [PubMed]
- Enzinger PC, Burtness BA, Niedzwiecki D, et al. CALGB 80403 (Alliance)/E1206: A Randomized Phase II Study of Three Chemotherapy Regimens Plus Cetuximab in Metastatic Esophageal and Gastroesophageal Junction Cancers. J Clin Oncol 2016;34:2736-42. [Crossref] [PubMed]
- Hutchinson RA, Adams RA, McArt DG, et al. Epidermal growth factor receptor immunohistochemistry: new opportunities in metastatic colorectal cancer. J Transl Med 2015;13:217. [Crossref] [PubMed]
- Argiris A, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet 2008;371:1695-709. [Crossref] [PubMed]
- Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 2002;62:7350-6. [PubMed]
- Roth AD, Fazio N, Stupp R, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol 2007;25:3217-23. [Crossref] [PubMed]
- Ajani JA, Fodor MB, Tjulandin SA, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol 2005;23:5660-7. [Crossref] [PubMed]
- Petrelli F, Borgonovo K, Barni S. The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: a systematic review and meta-analysis of published trials. Target Oncol 2013;8:173-81. [Crossref] [PubMed]
- Liu HB, Wu Y, Lv TF, et al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2013;8:e55128 [Crossref] [PubMed]
- Wöll E, Greil R, Eisterer W, et al. Oxaliplatin, irinotecan and cetuximab in advanced gastric cancer. A multicenter phase II trial (Gastric-2) of the Arbeitsgemeinschaft Medikamentose Tumortherapie (AGMT). Anticancer Res 2011;31:4439-43. [PubMed]
- Richards D, Kocs DM, Spira AI, et al. Results of docetaxel plus oxaliplatin (DOCOX) ± cetuximab in patients with metastatic gastric and/or gastroesophageal junction adenocarcinoma: results of a randomised Phase 2 study. Eur J Cancer 2013;49:2823-31. [Crossref] [PubMed]
- Tebbutt NC, Price TJ, Ferraro DA, et al. Panitumumab added to docetaxel, cisplatin and fluoropyrimidine in oesophagogastric cancer: ATTAX3 phase II trial. Br J Cancer 2016;114:505-9. [Crossref] [PubMed]
- Chan JA, Blaszkowsky LS, Enzinger PC, et al. A multicenter phase II trial of single-agent cetuximab in advanced esophageal and gastric adenocarcinoma. Ann Oncol 2011;22:1367-73. [Crossref] [PubMed]
- Lledo G, Huguet F, Chibaudel B, et al. Chemoradiotherapy with FOLFOX plus cetuximab in locally advanced oesophageal cancer: The GERCOR phase II trial ERaFOX. Eur J Cancer 2016;56:115-21. [Crossref] [PubMed]
- Lee MS, Mamon HJ, Hong TS, et al. Preoperative cetuximab, irinotecan, cisplatin, and radiation therapy for patients with locally advanced esophageal cancer. Oncologist 2013;18:281-7. [Crossref] [PubMed]
- Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol 2013;14:627-37. [Crossref] [PubMed]
- Okines AF, Ashley SE, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trial. J Clin Oncol 2010;28:3945-50. [Crossref] [PubMed]
- Suntharalingam M, Winter K, Ilson DH, et al. The initial report of RTOG 0436: A phase III trial evaluating the addition of cetuximab to paclitaxel, cisplatin, and radiation for patients with esophageal cancer treated without surgery. J Clin Oncol 2014;32:abstr 4007.


