
Trimming the fat in non-small cell lung cancer: a new small molecule inhibitor of acetyl-CoA carboxylase to target fatty acid synthesis
In the October 2016 issue of Nature Medicine, Svensson et al. demonstrate that acetyl-CoA carboxylase 1 (ACC1) is required for non-small cell lung cancer (NSCLC) growth in preclinical models and treatment with an allosteric ACC1 inhibitor, ND-646, suppresses NSCLC through fatty acid synthesis inhibition (1). This study provides answers to questions the cancer metabolism field has asked since the discovery that enzymes within the fatty acid synthesis pathway are overexpressed in cancer (2). Is de novo fatty acid synthesis required for tumors to develop and progress? And can enzymes in the pathway be successfully targeted for therapy?
Cellular fatty acid can be obtained from dietary sources or synthesized de novo. They participate in multiple cellular processes. They can be used as part of the cellular architecture by incorporation into phospholipid. They can be stored as energy in triglycerides and can serve as a source of energy through β-oxidation in the mitochondria. They can also serve as precursors to signaling molecules and can be added to proteins as post-translational modifications to regulate localization and function. Depending on cellular location, type, and metabolic status, fatty acids may be partitioned according to cellular context. Because of this, fatty acid synthesis is segregated into a multi-step process to provide multiple levels of regulation and to facilitate the entry or exit of substrates and intermediates as needed.
ACC1 is a cytosolic, multifunctional enzyme that catalyzes the ATP and biotin-dependent conversion of acetyl-CoA to malonyl-CoA via carboxylation in the first and rate-limiting step of de novo fatty acid synthesis. When active, ACC1 is found in a polymerized form (3) (Figure 1A). The carboxylation of acetyl-CoA to malonyl-CoA by ACC1 starts at the biotin carboxylase (BC) domain where bicarbonate donates carbon dioxide to carboxylate biotin. The biotin carboxyl carrier protein domain then facilitates transfer of the carboxy group to the carboxy transferase domain, where acetyl-CoA is carboxylated to malonyl-CoA (Figure 1A). Seven moles of malonyl-CoA are then combined with one mole of acetyl-CoA by fatty acid synthase (FASN) to make palmitate, using nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor. ACC1 is one of the two mammalian isoforms of the acetyl-CoA carboxylases, the other being acetyl-CoA carboxylase 2 (ACC2). ACC2 contains an N-terminal mitochondrial targeting sequence which ACC1 lacks (4). The catalytic subunits of the ACC proteins are homologous and both enzymes perform the same function (4,5). However the pools of malonyl-CoA created by ACC1 and ACC2 are thought to be distinct and participate in different processes. Malonyl-CoA generated by ACC1 is used primarily for de novo fatty acid synthesis (6), whereas the malonyl-CoA generated by ACC2 regulates fatty acid oxidation through modulation of carnitine palmitoyltransferase I (CPT-1) (5).
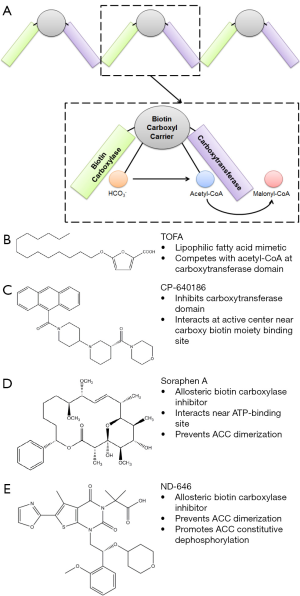
Increased ACC1 expression is associated with several cancers including breast (7,8), liver (7), lung (9), and prostate (10). The ACCs are subject to a variety of regulations including transcriptional regulation by the sterol regulatory element binding proteins (SREBPs) and the carbohydrate response element binding protein (ChREBP), allosteric regulation by citrate and fatty acid, and inactivating phosphorylation by AMP-dependent protein kinase (AMPK). In addition, several non-isoform specific pharmacological inhibitors such as CP-640186, 5-(tetradecyloxy)-2-furoic acid (TOFA), soraphen A, and, now, ND-646 have been described (Figure 1B-E) (1,7,11-13). Despite the abundance of evidence linking ACC1 to cancer, there has been no definitive data demonstrating that it is required for tumor growth and no ACC1 inhibitors suitable for cancer therapy have previously been reported. The facts that the ACCs are subject to such extensive biological regulation and that increased expression of ACC1 has been described in several cancers supports the widely held hypothesis that ACC1 is indeed vital in cancer development and progression and thus represents a viable therapeutic target.
Because de novo synthesized fatty acid can be used in many cellular processes, it is not surprising that blockade of ACC activity has been associated with a variety of downstream mechanisms. For example, it has been demonstrated that siRNA mediated inhibition of ACC1 or pharmacological inhibition of ACC results in inhibited proliferation and cell cycle arrest and/or cell death in several cancer cell lines (7,10,14-18). In some cases the results appear to be more dramatic than others, owing to many factors including differences in cell lines or tissue of origin being studied, the level of knockdown or inhibition, or some other host of factors. What is striking about the totality of this work is that although there is a clear suggestion that ACC1 is required for tumor growth, there has yet to be a definitive demonstration that ACC is required for tumor growth. The article by Svensson and colleagues provides that evidence. There is also some question of whether the two isoforms of ACC provide separate or distinct pools of malonyl-CoA, and what those pools can be used for. Genetic inactivation of ACC1 in the mouse liver reduces hepatic malonyl-CoA levels, suggesting ACC2 cannot compensate for the loss of ACC1 (6). Alternatively, there is evidence to suggest ACC2 can compensate for the loss of ACC1 in mouse liver as ACC2 activity is increased in response to genetic ACC1 inactivation and malonyl-CoA levels are maintained (11). Because of this, it reasons that ACC targeted therapies may be best suited if they inhibit both isoforms. Svensson and colleagues describe a compound in ND-646 that has such ability.
In their article, Svensson et al. accomplish several important goals. They provide the first evidence that ACC1, and by extension fatty acid synthesis, is required for proliferation and survival of tumor cells in vitro and in vivo. While many groups have demonstrated that ACC and other fatty acid synthesis enzymes are required in vitro, using CRISPR technology, Svensson et al. generated clones lacking ACC1 that were unable to grow in vivo. In NSCLC, the lack of ACC1 induces apoptosis through an ER stress associated mechanism. This is in-line with literature connecting lipid synthesis and ER homeostasis (19). They further demonstrate that ND-646 acts via a unique mechanism. First, ND-646 promotes constitutive dephosphorylation of ACC by interacting with Arg172 of ACC1 (Arg277 in ACC2) which interacts with AMPK-phosphorylated serines (Ser79 in ACC1, Ser221 in ACC2). ND-646 also disrupts ACC dimerization and activity by interacting with residues within ACC’s dimerization site in the BC domain (Figure 1A). In doing so, ACC1 is inactivated by loss of dimerization rather than by AMPK-mediated inhibition, but in a manner that is unique relative to the other inhibitors described in Figure 1B-E. It could also be the reason that ND-646 is so effective at blocking tumor growth. The data also demonstrates that ND-646 is not isoform specific and work on both ACC1 and ACC2. Importantly, ND-646 reduces tumor fatty acid levels, indicating the drug does in fact hit and inhibit its target in tumors. Moreover, ND-646 appears to also reduce circulating fatty acids. While the mechanism behind this is not clear, the ND-646 predecessor compound, ND-630, was developed to inhibit hepatic ACC, so the effect could be related to liver fatty acid metabolism. This may be of little concern in terms of negative side effects in that ACC1 appears to not be required for normal liver function (6).
As the development of ND-646 and other ACC inhibitors for cancer therapy progresses, several issues should be considered. Because cells can utilize dietary fatty acid there is a possibility that cells can appropriate dietary fat to moderate the effects of fatty acid synthesis inhibitors. In fact, this article illustrates that cells deficient in ACC1 can be supported by supplementation with palmitate. Performing studies where dietary fat is manipulated, either the amount or type of dietary fat, in vivo could lead to strategies that improve therapeutic efficacy. Whether ACC2 is redundant for ACC1, or vice versa, should also be considered in tumors where ACC2 is highly expressed, which is not the case in NSCLC. Although ND-646 is a non-isoenzyme selective compound, understanding the contribution of each isoform of ACC could be of biological significance. As stated by the authors, ND-646, and perhaps future ACC inhibitors, could have impact not just by inhibition of fatty acid synthesis, but also by alterations of fatty acid oxidation as well because of their ability to inhibit both ACC isoforms. In addition, the mechanism by which ND-646 promotes the dephosphorylated state of ACC suggests that it could be more beneficial in some tumors than others, in particular as it relates to LKB1 status owing to LKB1’s ability to activate AMPK thus leading to the inactivating phosphorylation of ACC. Lastly, in the future it will be interesting to employ 11C-acetate PET, which has been correlated with fatty acid synthesis, to ascertain how long after administration ND-646 blocks ACC and fatty acid synthesis, perhaps leading to enhanced dosing schedules (20). In summary, the work by the Shaw group and collaborators represents an important step in the development of ACC inhibitors for clinical cancer therapy and sets the stage for an exciting time for metabolism research and for those interested in targeting metabolic enzymes in general, and the fatty acid synthesis pathway specifically.
Acknowledgments
Funding: This work was supported by R01 CA161503 to SJK.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Lichao Sun (State Key Laboratory of Molecular Oncology, National Cancer Center (NCC)/Cancer Hospital, Chinese Academy of Medical Sciences (CAMS), Peking Union Medical College, Beijing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Svensson RU, Parker SJ, Eichner LJ, et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med 2016;22:1108-19. [Crossref] [PubMed]
- Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care 2006;9:358-65. [Crossref] [PubMed]
- Beaty NB, Lane MD. The polymerization of acetyl-CoA carboxylase. J Biol Chem 1983;258:13051-5. [PubMed]
- Abu-Elheiga L, Brinkley WR, Zhong L, et al. The subcellular localization of acetyl-CoA carboxylase 2. Proc Natl Acad Sci U S A 2000;97:1444-9. [Crossref] [PubMed]
- Kim KH. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu Rev Nutr 1997;17:77-99. [Crossref] [PubMed]
- Mao J, DeMayo FJ, Li H, et al. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci U S A 2006;103:8552-7. [Crossref] [PubMed]
- Wang C, Xu C, Sun M, et al. Acetyl-CoA carboxylase-alpha inhibitor TOFA induces human cancer cell apoptosis. Biochem Biophys Res Commun 2009;385:302-6. [Crossref] [PubMed]
- Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition 2000;16:202-8. [Crossref] [PubMed]
- Conde E, Suarez-Gauthier A, García-García E, et al. Specific pattern of LKB1 and phospho-acetyl-CoA carboxylase protein immunostaining in human normal tissues and lung carcinomas. Hum Pathol 2007;38:1351-60. [Crossref] [PubMed]
- Brusselmans K, De Schrijver E, Verhoeven G, et al. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res 2005;65:6719-25. [Crossref] [PubMed]
- Harada N, Oda Z, Hara Y, et al. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol 2007;27:1881-8. [Crossref] [PubMed]
- Beckers A, Organe S, Timmermans L, et al. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res 2007;67:8180-7. [Crossref] [PubMed]
- Tong L, Harwood HJ Jr. Acetyl-coenzyme A carboxylases: versatile targets for drug discovery. J Cell Biochem 2006;99:1476-88. [Crossref] [PubMed]
- Zhan Y, Ginanni N, Tota MR, et al. Control of cell growth and survival by enzymes of the fatty acid synthesis pathway in HCT-116 colon cancer cells. Clin Cancer Res 2008;14:5735-42. [Crossref] [PubMed]
- Chajès V, Cambot M, Moreau K, et al. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res 2006;66:5287-94. [Crossref] [PubMed]
- Knowles LM, Yang C, Osterman A, et al. Inhibition of fatty-acid synthase induces caspase-8-mediated tumor cell apoptosis by up-regulating DDIT4. J Biol Chem 2008;283:31378-84. [Crossref] [PubMed]
- Zhou W, Han WF, Landree LE, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res 2007;67:2964-71. [Crossref] [PubMed]
- Zhou W, Simpson PJ, McFadden JM, et al. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res 2003;63:7330-7. [PubMed]
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 2007;7:763-77. [Crossref] [PubMed]
- Vāvere AL, Kridel SJ, Wheeler FB, et al. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J Nucl Med 2008;49:327-34. [Crossref] [PubMed]


