Advances in clinical immunotherapy of melanoma
Background
Immunity and cancer
The concept of directing the immune system toward malignant tumors as an anti-cancer strategy has stimulated scientific inquiry, experimentation, and clinical investigation for more than a century. The now infamous “Coley’s toxin” is the first known clinical success of tumor regression from local stimulation of immunity using bacteria injected directly into malignant lesions. Via this technique, in 1891 Coley reported a 10% cure rate in soft-tissue sarcomas (1-3). Recognition of the interplay between cancer and the immune system was later asserted by Paul Erlich, the 1909 Nobel Laureate physician and scientist who proposed cancer developed in vivo and that the immune system was able to both recognize and protect against it (4). In subsequent decades the mainstays of cancer treatment for solid malignancies evolved toward surgery, radiation, and infusions of cytotoxic chemotherapy, and the role of the immune system remained nascent until the 1970s, when T-cells were identified and thought to be the protective cells of Dr. Ehrlich’s theory. Nearly three decades later, the landmark work on immune checkpoints published by Allison and colleagues in 1996 served as a tipping point for the extraordinary advances seen in immuno-oncology today (5).
Melanoma—a frontrunner in the immunotherapy movement
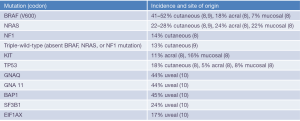
Cutaneous melanoma is the most lethal form of skin cancer, and while not the most common cancer in the United States (6), it was among the deadliest prior to immunotherapy, with estimated 5-year overall survival (OS) of only 18% (7). Distinct genetic and molecular derangements exist among the different sites of origin and subtypes (Figure 1), and while all forms of melanoma have been relatively resistant to traditional cytotoxic chemotherapy, cutaneous melanoma has been the platform from which modern era immune therapies have sprung. Initial research on tumor infiltrating lymphocyte (TIL) therapy, using T-lymphocytes extracted from melanoma tumors and then re-infused into patients with metastatic disease, showed an impressive response rate of 34% (11). Later co-administration with IL-2 showed enhanced responses to TIL, and IL-2 administered alone also demonstrated efficacy including durable complete responses (CR) in approximately 5% of patients (12,13). Enthusiasm for these early successes with cytokine and cell transfer therapies was tempered by intensive dosing schedules, substantial toxicity, and logistical hurdles which limited use to medically fit patients at experienced treatment centers. Within a short time, however, advancements have enabled many patients to benefit both in the academic and community settings, with improved efficacy and toxicity profiles.
Ushering in a new era—checkpoint blockade immune therapies
A difference in ex vivo and in vivo cytotoxic effects of T-cells led to recognition of co-opted mechanisms used by malignant cells for immune evasion. While immune recognition of cancers as “non-self” first requires T cell receptor engagement to major histocompatibility complex (MHC) containing antigenic peptide, additional costimulatory signals are necessary for T cell activation (14). The most important of these costimulatory signals appears to be provided by the interaction of CD28 on T cells with its primary ligands B7-1 (CD80) and B7-2 (CD86) on the surface of specialized antigen-presenting cells (APCs), which is inhibited when cytotoxic T-lymphocyte antigen 4 (CTLA-4) binds to CD80, serving as an “immune checkpoint” (15). Ipilimumab, an IgG1-kappa monoclonal antibody (mAb) directed against CTLA-4, interrupts this T-cell inhibitory pathway and now holds FDA-approval in both the adjuvant and metastatic treatment settings (16-18).
Another co-stimulatory receptor of importance is programmed death receptor 1 (PD-1), which is expressed by activated T-cells and mediates immune response to limit extensive tissue damage. PD-1 is upregulated on T-cells in an inflammatory environment and binds to its ligands PD-L1 or PD-L2. PD-L1 is expressed on a variety of tumors, including melanoma, and via binding to PD-1 can suppress T-cell proliferation, cytokine release, and cytotoxicity (19). Pembrolizumab and nivolumab are both IgG4-kappa anti-PD-1 monoclonal antibodies that abrogate T-cell binding to PD-L1 and PD-L2, and have demonstrated superior progression-free survival (PFS) and overall survival (OS) outcomes in melanoma both naïve to, and refractory to, ipilimumab therapy (20,21). Pembrolizumab and nivolumab are both FDA approved as single-line therapies, and nivolumab is also FDA-approved in combination with ipilimumab.
The dramatic responses to checkpoint blockade, efficacy in a variety of malignancies, and relative ease of administration have appealed to both patients and physicians regarding their use. However, checkpoint blockade can induce immune-mediated toxicities—inflammatory cascades of variable degrees in any organ. Use of guideline recommendations for management of toxic effects, the so-called immune-related adverse events (irAEs), is key to preventing catastrophic outcomes (22-25). Considering the potential for severe toxicities from any immune therapy in patient selection is fundamental to uphold the “primum, non nocere” tenet of medical practice.
Full circle—oncolytic vaccine therapy
Harkening to “Coley’s Toxin” of the nineteenth century is the first-in-class FDA-approved injectable oncolytic virus, Talimogene laherparepvec (T-VEC), a herpes simplex virus (HSV) type 1 designed for selective replication within tumors that can induce an anti-tumor immune response. Remarkably, both local and distant immune activation can occur with intralesional or intranodal injection of T-VEC (26). Other approaches of cancer vaccination in melanoma are based on targeting tumor-associated antigens, but none has yet led to FDA-approval (27). Clinical trials of TVEC in combination with immune checkpoint antibodies are currently underway (28).
Case scenarios
Case 1
In 2004, a 32-year-old man presents to clinic with a cough for 2 months. He underwent wide local excision of an ulcerated 3.8 mm thick melanoma from his trunk 2 years prior. A left axillary sentinel node was positive with a 3-mm focus of melanoma. He underwent complete left axillary node dissection revealing no other nodal metastasis (final Stage IIIB, pT3bN1a). He initiated adjuvant interferon alpha but stopped after 6 months due to intolerance. For the cough, a chest X-ray was done which revealed numerous pulmonary nodules. Further radiographic staging with computed tomography (CT) of the chest, abdomen and pelvis revealed additional numerous lesions in the liver. CT-guided lung biopsy confirmed metastatic melanoma. He proceeded to treatment with IL-2 which resulted in a partial response (PR) for 3 years. Upon recurrent disease in the liver, he receives TIL therapy resulting in complete remission.
Case in context: management of metastatic melanoma prior to checkpoint blockade
In 2004, the median survival of patients diagnosed with metastatic melanoma to visceral organs was 5.1 months (29), owing to the limited systemic therapies available at the time, either dacarbazine (approved in 1975) or high-dose IL-2 (approved in 1998). Non-FDA approved therapies included a clinical trial, other chemotherapies, or adoptive T-Cell therapy (ACT), the last of which is a reasonable (and potentially curative) option in a young, fit patient. This particular case highlights the paucity of therapies available in the era before contemporary checkpoint blockade and targeted therapy.
Dacarbazine
Dacarbazine (DTIC) is an alkylating agent that received FDA approval for use in metastatic melanoma in 1975 despite lack of randomized trials demonstrating benefit over placebo or best supportive care. Nevertheless, it became a benchmark for other systemic therapies (30), and had modest overall response rates (ORR) of 15–28%, CR in 3–5%, and response durations up to 6 months (31-34). In this modern era of immune therapy, it is unclear whether any role for the traditional cytotoxic chemotherapy, as monotherapy, exists. The combination of chemotherapy and immune therapy or targeted therapy has been studied and will be addressed later in this review.
Interleukin-2
The cytokine and T-cell growth factor, IL-2, is considered to be the first effective anti-cancer immune therapy. The concept of using IL-2 in cancer came about through discovery in the 1970s of its ability to stimulate growth and differentiation of CD8+ cytotoxic lymphocytes, its role in maintenance of CD4+ regulatory T cells, and in differentiation of CD4+ T cells. Administration of IL-2 in murine cancer models was eventually translated into humans with success in both renal cell carcinoma and melanoma. The success seen in murine models was not replicated in humans until the high-dose bolus regimen was used that has become the standard formula to this day (12,35-37).
The first series of patients receiving high-dose IL-2 included 7 patients with melanoma and 3 with metastatic renal cell cancer. Tumor regression occurred in 4 of the 7 patients with melanoma and all 3 patients with kidney cancer. Toxicity included a capillary leak syndrome resulting in fluid extravasation into visceral organs. This syndrome caused organ dysfunction that returned to baseline at conclusion of treatment (12). Based on subsequent multi-institutional studies of high-dose IL-2 in renal cell carcinoma showing an ORR of 14% and 5% CR rate, IL-2 became the first FDA-approved immune therapy in cancer in 1992 (38). Trials across 22 institutions using high-dose bolus IL-2 in a total of 270 patients with metastatic melanoma were conducted between 1985 and 1993. These demonstrated an ORR of 16% with 17 CRs (6%) and 26 PRs (10%) (39). These results and the extended durability seen among complete responders led to FDA-approval of high-dose IL-2 in metastatic melanoma in 1998. During the early clinical trials of IL-2 there were treatment-related deaths near 2–4%, but mortality has dropped to <1% as clinicians gained experience with management of the complexities of administration and inherent toxicities from this immune-based cancer therapy.
An updated analysis of IL-2 outcomes through July 2015 from the PROCLAIMSM registry included 170 patients with metastatic melanoma treated between 2005 and 2012. CR was observed in 5%, PR in 10%, stable disease (SD) in 22%, and 63% had progressive disease (PD). The median overall survival (mOS) for these patients was 19.6 months, with a median follow-up of 43.1 months. The mOS was not reached for patients achieving CR or PR, and was 33.4 months for patients with SD (13). These data support consideration for IL-2 among eligible patients, even in the modern era of immunotherapy, as long-term survival outcomes can be seen in patients with disease response.
ACT
As described earlier in this review, successful outcomes in patients with metastatic melanoma using infusion of autologous ex vivo expanded TIL were first published in 1988 (11). The original method of ACT in melanoma involved extracting TILs from a resected tumor followed by ex vivo expansion in culture and finally re-infusion with co-administration of IL-2. The use of IL-2 along with reinfusion of TILs produced superior in vivo lymphocyte expansion (37). In one of the earliest trials of TIL therapy, out of 35 pre-treated patients (34 of whom had prior treatment with IL-2), 18 (51%) demonstrated ORR. Among responders, 3 had a CR and 15 had a mixed or minor response. Since initial trials, lymphodepletion has improved response rates to more than 50% (40-42). Use of a lymphodepleting regimen (cyclophosphamide and fludarabine) eliminates T regulatory cells and non-cytotoxic lymphocytes, which compete with transferred cells for homeostatic cytokines. A recent randomized prospective trial investigated the addition of total body irradiation (TBI) to lymphodepletion. One hundred and one patients with metastatic melanoma, including 76 with M1c disease, were randomly assigned to receive nonmyeloablative chemotherapy with or without 1,200 cGy TBI prior to transfer of TIL. The primary endpoints were CR (as defined by RECIST 1.0) and OS. CR rates were 24% in both groups, and OS was also similar (median OS, 38.2 vs. 36.6 months). With a median potential follow-up of 40.9 months, only one of the 24 patients achieving CR recurred. This trial showed TBI does not provide additional benefit to nonmyeloablative chemotherapy (43).
Future directions for use of TIL therapy include identifying predictive biomarkers for successful TIL expansion and clinical response, combination and sequencing with other immune therapies, establishment of consistent protocols based on phase II/III clinical trials, and infusion of lymphocytes with enhanced tumor killing effects through using chimeric, genetically modified, or antigen-expanded autologous lymphocyte populations (44). While significant success has occurred in patients with metastatic melanoma and TIL therapy, limitations exist. The intensity of therapy mirrors stem cell transplant and requires inpatient management of side effects before and after TIL infusion. Logistical limitations include the need for a specialized center for TIL acquisition, engineering, expansion and infusion, and institutional experience with high-dose IL-2. While TIL therapy has proved to be an impressive treatment option, it has not become a standard of care due to these complexities.
Case 2
The same patient as in Case 1 returns in 2012 at the age of 40. He now has relapsed disease with cutaneous and pulmonary nodules, and is treated with four doses of ipilimumab IV every three weeks. Following treatment, several pulmonary and cutaneous nodules regress, others are stable in size, and no new lesions have appeared 4 years later.
Case in context: management of metastatic melanoma in the era of immune checkpoint blockade
In March of 2011, ipilimumab was granted FDA approval for use in patients with unresectable or metastatic melanoma, following results of a Phase III trial comparing ipilimumab alone, ipilimumab plus gp100 vaccine, and gp100 vaccine plus placebo in 676 patients with metastatic melanoma (16). The mOS was 10.0 months among patients receiving ipilimumab plus gp100, as compared with 6.4 months among patients receiving gp100 alone (hazard ratio for death, 0.68; P<0.001). The mOS with ipilimumab alone was 10.1 months, thus no difference in OS was detected between the ipilimumab groups. Immune-related adverse events, most commonly affecting the skin and gastrointestinal tract, occurred in approximately 60% of patients treated with ipilimumab and 32% of patients treated with gp100 alone. Grade 3 or 4 irAEs occurred in 10% to 15% of patients treated with ipilimumab and in 3% treated with gp100 alone. Up to 31% of patients receiving ipilimumab developed diarrhea. This was typically controlled with corticosteroids, however, four patients required infliximab (anti-tumor necrosis factor alpha antibody) for grade 3 or higher colitis. Eight patients required hormone replacement for endocrine immune-related adverse events.
OS data from patients treated with ipilimumab for unresectable or metastatic melanoma were pooled from ten prospective and two retrospective studies with a separate analysis of patients treated on an expanded access program (EAP). Among 1,861 patients treated on study the mOS was 11.4 months and after up to 10 years in follow-up the survival curve was seen to plateau near 3 years. Among 2,985 patients who received ipilimumab from an EAP, the median OS was 9.5 months with a similar 3-year survival curve plateau. Remarkably, the long-term survival benefit from ipilimumab appeared to be independent of prior therapy (45).
Case 3
A 62-year-old woman with no prior history of melanoma presents in 2016 with a bleeding 4 cm mass proximal to her elbow. Biopsy reveals malignant melanoma. Radiographic staging with whole-body positron emission tomography (PET) and brain magnetic resonance imaging (MRI) reveals several scattered hepatic lesions and bilateral subcentimeter pulmonary nodules. No intracranial lesions are seen. Liver biopsy confirms metastatic melanoma. She begins combination immunotherapy with ipilimumab and nivolumab, and completes 3 of 4 induction cycles prior to discontinuation for grade 2 rash and grade 3 hepatotoxicity. After a 3-week course of 1 mg/kg corticosteroids, symptoms and lab derangements abate and she resumes monotherapy with nivolumab.
Case in context: management of metastatic/unresectable melanoma in the era of anti-CTLA-4 and anti-PD-1 antibody therapy
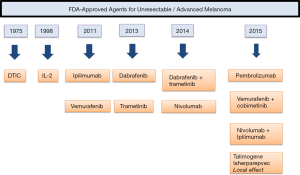
For this patient with de novo metastatic disease who is otherwise healthy, single-agent checkpoint blockade or combination treatment with ipilimumab and nivolumab are both reasonable first-line therapies (Figure 2).
Anti-PD-1 antibodies: nivolumab and pembrolizumab
Both PD-1 antagonist antibodies, nivolumab and pembrolizumab, have been granted FDA-approval for the treatment of metastatic melanoma. Each has demonstrated superior ORR, PFS, and OS compared to chemotherapy and in treatment naïve patients to ipilimumab. In an effort to overcome mechanisms of resistance, and improve durable responses, combination of ipilimumab with each PD-1 antibody has been of particular interest.
(I) Pembrolizumab
The phase II, KEYNOTE-002, trial compared pembrolizumab with investigator-choice chemotherapy (ICC) in 540 patients with ipilimumab refractory metastatic melanoma. Patients were randomized 1:1:1 to pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or ICC. ORRs were 25% and 21% for the pembrolizumab 10 and 3 mg/kg groups, respectively, versus 4% for ICC. Six-month PFS was 34% in the pembrolizumab 2 mg/kg group, 38% in the 10 mg/kg group, and 16% in the ICC group. Median PFS in the pembrolizumab arms were 5.6 and 5.4 months versus 3.6 months for ICC. Unfortunately, OS data were not interpretable due to crossover from the chemotherapy group to the pembrolizumab group (47).
The phase III, KEYNOTE-006, trial randomized 834 patients to receive different dosing frequencies of pembrolizumab 10 mg/kg versus standard-dose ipilimumab (3 mg/kg every 3 weeks for 4 cycles) in patients with metastatic melanoma who had received no more than one prior line of therapy (48). The ORR was similar with pembrolizumab administered every 2 weeks (33.7%) and every 3 weeks (32.9%), but superior compared to ipilimumab (11.9%). Updated survival analysis showed the mOS was not reached for pembrolizumab versus 16.0 months with ipilimumab, with an estimated 24-month OS rate of 55% (compared to 43% for ipilimumab) (P=0.0008) (49).
An ongoing trial, KEYNOTE-029, is looking at pembrolizumab 2 mg/kg every 3 weeks combined with low-dose ipilimumab (1 mg/kg) every 3 weeks for 4 doses, then maintenance pembrolizumab up to 2 years. At a median follow-up of 6.4 months, 79 pts (74%) received all 4 doses of ipilimumab and 73 pts (68%) remained on pembrolizumab, with an ORR of 51% (9% CR and 42% PR) (50) (NCT02089685).
(II) Nivolumab
The phase I dose-escalation trial of nivolumab assessed 0.1, 0.3, 1, 3, and 10 mg/kg doses administered IV every 2 weeks (for up to 96 weeks) in 107 patients with advanced melanoma that had received 2 to 5 prior lines of therapy, not including anti-CTLA-4. Median OS was 16.8 months, with 1- and 2-year survival rates of 62% and 43%, respectively. Median PFS was 2 years (51). Recently updated survival data reported 5-year OS of 34% (95% CI: 24.6, 42.9) and mOS of 17.3 months (95% CI: 12.5, 37.8), with survival appearing to plateau near 48 months (52).
The CheckMate 066 trial was a Phase III study of nivolumab 3 mg/kg versus dacarbazine (DTIC) in 418 patients with treatment-naïve, BRAF wild-type advanced melanoma. At 1 and 2 years, OS was 72.9% and 57.7% in the nivolumab group and 42.1% and 26.7% in the DTIC group (P<0.001). The median PFS was 5.1 months in the nivolumab group versus 2.2 months in the DTIC group (P<0.001), with ORR 40.0% with nivolumab versus 13.9% with DTIC (P<0.001) (53).
The phase III, CheckMate 037, trial compared nivolumab and investigator choice of chemotherapy (ICC) in 405 patients with advanced melanoma. Patients had to have received prior anti-CTLA-4 therapy and, if BRAF mutation-positive, a BRAF inhibitor. Patients were randomized 2:1 to nivolumab 3 mg/kg every 2 weeks or ICC. ORR in the nivolumab group was 31.7% compared to 10.6% in the ICC group, again confirming superiority of checkpoint blockade to standard-of-care chemotherapy in pre-treated patients. Updated survival data, including OS, are eagerly awaited (54).
The phase II, CheckMate 172, trial also looked at the efficacy of nivolumab in patients with more advanced disease, including 307 patients with poor prognostic factors which would have excluded them from other trials (such as brain metastases and ECOG performance status of 2). All patients had prior treatment with ipilimumab. Nivolumab was given at 3 mg/kg every 2 weeks for up to 2 years. Overall, 256 (84%) patients received at least 4 doses of nivolumab, while 241 (79%) received >4 doses. Twelve-week ORR was 29.5%, with 3% CR, 26% PR, and 34% SD, with a low incidence of Grade 3/4 adverse events (11%) (55) (NCT02156804).
Therapeutic sequence with CTLA-4 and PD-1
The optimal sequencing of checkpoint blockade is being investigated. The phase II, CheckMate 064, trial sought to determine if differences in ORR, PFS, and OS occur relative to sequencing of nivolumab and ipilimumab in 140 patients with advanced melanoma. Patients were randomized 1:1 to receive sequential induction treatment with nivolumab 3 mg/kg every two weeks for 6 doses followed by ipilimumab 3 mg/kg every 3 weeks for 4 doses (Cohort A) or ipilimumab followed by nivolumab with the same dosing strategy (Cohort B). Following induction, both cohorts received maintenance nivolumab. ORR in Cohort A was 54% versus 31% in Cohort B, with more CRs seen in Cohort A vs. B (11% vs. 6%). A significant difference in OS was observed between Cohorts A and B (HR: 0.48; 95% CI: 0.29, 0.80; P=0.0041), with a median OS not reached vs. 16.9 months, and 1-year OS rates of 76% and 54%, respectively. Response was noted to be higher in patients with PD-L1 expression ≥5% in both treatment groups, which may point to the importance of biomarker status when considering sequence of therapy (56).
Combination of anti-CTLA-4 and anti-PD-1
The landmark phase III trial, CheckMate 067, investigating combination anti-CTLA-4 and anti-PD-1 therapies in previously untreated patients with advanced melanoma has produced the most dramatic results seen in the history of melanoma. The trial included 945 patients randomized in a 1:1:1 fashion to nivolumab 3 mg/kg every two weeks; nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for 4 doses, followed by maintenance nivolumab every two weeks; or ipilimumab 3 mg/kg every three weeks for 4 doses. PFS and OS were co-primary endpoints; of particular interest was the secondary endpoint of efficacy by PD-L1 expression. ORRs were 57.6%, 43.7%, and 19% in the combination, nivolumab, and ipilimumab arms, respectively, and the initial survival analysis demonstrated median PFS 6.9 months in the nivolumab group, 11.5 months in the nivolumab plus ipilimumab group, and 2.9 months in the ipilimumab group. Combination therapy yielded a 58% risk reduction for progression that was statistically significant. At ≥18 months of follow-up, median duration of response (DOR) in responders treated with combination therapy has not been reached, and was 22.3 and 14.4 months in the nivolumab and ipilimumab responders, respectively. Median PFS was also longer with combination therapy regardless of PD-L1 expression or BRAF mutation status (57).
While combination therapy yielded remarkable clinical results, the outcomes were offset by a higher rate of immune-mediated toxicities. Treatment-related adverse events of any grade that led to discontinuation of the study drug occurred in 7.7% of the patients in the nivolumab group, 36.4% of those in the nivolumab-plus-ipilimumab group, and 14.8% of those in the ipilimumab group, with the most common events being diarrhea (in 1.9%, 8.3%, and 4.5%, respectively) and colitis (in 0.6%, 8.3%, and 7.7%, respectively). One death due to toxic effects of the study drug was reported in the nivolumab group (neutropenia) and one in the ipilimumab group (cardiac arrest), but none were reported in the nivolumab-plus-ipilimumab group. Immune modulatory agents, including topical agents, to manage adverse events were used in 47.0% of the patients in the nivolumab group, 83.4% of those in the nivolumab-plus-ipilimumab group, and 55.9% of those in the ipilimumab group, with secondary immunosuppressive agents (e.g., infliximab) used in 0.6%, 6.1%, and 5.1% of the patients, respectively.
Pembrolizumab has also demonstrated efficacy in combination with ipilimumab. The KEYNOTE 029 trial enrolled 153 patients with advanced melanoma and administered pembrolizumab 2 mg/kg plus ipilimumab 1 mg/kg every three weeks for 4 doses, followed by maintenance pembrolizumab 2 mg/kg every three weeks for up to 24 months unless stopped early for intolerable toxicity or progression. Patient characteristics included 18% with elevated LDH, 55% with Stage M1c disease, 36% were BRAF mutant, 13% with more than 1 prior line of therapy, and 12% previously treated with BRAF + MEK inhibitors. Notably, 84% had PD-L1+ tumors (cutoff ≥1%). Preliminary efficacy data showed ORR of 51%, with 9% CR (n=5) and 42% PR (n=56). Combination therapy with a lower dose of ipilimumab had a more favorable safety profile, where 38% of patients had ≥1 grade 3–4 drug-related AE (DRAEs) but 68% of these had resolved by data cutoff. DRAEs led to discontinuation of combination therapy in 8% (n=9), ipilimumab alone in 10% (n=11), and pembrolizumab alone in 4% (n=4). There were no treatment-related deaths. Immune-mediated AEs of any grade and grade 3–4 severity occurred in 53% and 20% of patients, respectively (50).
Optimal dosing of checkpoint blockade, particularly ipilimumab, that achieves maximum efficacy with minimal toxicity remains unclear. The KEYNOTE 029 trial using lower dose ipilimumab than CheckMate 067 preliminarily shows similar efficacy with fewer patients discontinuing therapy for DRAEs.
The toxicities associated with checkpoint blockade antibodies range from very mild to potentially life threatening. They can involve any organ, but most commonly affect the skin, gastrointestinal tract, liver, and endocrine system. Toxicity management typically involves symptomatic treatment, delay in therapy, or in the case of grade 3/4 toxicity, cessation of therapy and administration of high-dose corticosteroids. Infliximab is effective in severe colitis (58), and mycophenolate mofetil in the case of severe hepatitis (59). Immune suppressive therapy use ranges 2 weeks to 2 months and often a full recovery is achieved, with the exception of endocrine irAEs which often require lifelong hormone replacement. Deaths have occurred from irAEs, most commonly from intestinal perforation in patients getting ipilimumab (60). Multiple publications offer expert recommendations on treatment of toxicities from checkpoint blockade (22-25,61-63).
Case 4
A 58-year-old man with a history of stage IIA (pT2bN0) resected melanoma presents with hemiparesis, dysarthria, and confusion. Staging imaging reveals nine brain lesions (2 are >3 cm) and pulmonary nodules. The largest brain lesions are resected and he is placed on corticosteroids. Resolution of neurological symptoms occurs rapidly and he declines post-operative radiation. Molecular testing of the brain tumor reveals BRAF V600E mutation. He is started on BRAF and MEK combined inhibitor therapy resulting in significant diminution of brain lesions. He is tapered off of corticosteroids, treated for 3 months with molecular targeted therapies and transitions to combination checkpoint blockade. Disease has been stable for 8 months with continuation of anti-PD-1 therapy.
Case in context: management of melanoma brain metastases
Intracranial metastases occur in up to 75% of patients and pose significant challenges (64). Rapid control of tumors that are high-risk for hemorrhage is critical. Tools including surgical decompression, stereotactic radiosurgery, whole-brain radiation, or systemic therapy should be considered. Melanoma oncologists utilize a multidisciplinary approach toward balancing maximal tumor regression with minimal neurocognitive sequelae.
Tumor regression can occur quickly after administration of molecular targeted therapies in patients with BRAF V600E mutations, although may take as long as 12 weeks with immunotherapy. Furthermore, administration of immune therapy may be delayed during courses of corticosteroids for cerebral edema. Several early trials have shown efficacy in treating metastatic brain lesions. Retrospective and early phase studies evaluating concurrent or sequential treatment with radiation and checkpoint blockade are ongoing.
A phase II open-label trial established safety and efficacy of treatment with ipilimumab in patients with melanoma brain metastases. Patients were divided in two groups: cohort A (51 neurologically asymptomatic patients, not receiving corticosteroids) and cohort B (21 patients with neurologic symptoms on a stable corticosteroid dose) and received ipilimumab (10 mg/kg every 3 weeks for four doses). If patients maintained clinical stability at 24 weeks, they continued receiving ipilimumab every 12 weeks. Patients were assessed after 12 weeks for the primary endpoint of disease control (CR, PR, or SD), which was greater in the asymptomatic cohort (18%, 95% CI: 8–31 versus 5%, 0.1–24). The most common AEs were diarrhea, fatigue, nausea, and rash, with one death occurring from immune-related colitis. Grade 4 confusion occurred in one patient from each cohort. No new AEs were noted and proportion of AEs was similar to trials in patients without brain metastases (65).
Early analysis of an ongoing phase 2 trial of 52 patients (18 with melanoma, 34 with non-small cell lung cancer) with untreated or progressive brain metastases after radiation showed activity of pembrolizumab (10 mg/kg every 2 weeks until progression) and tolerability. No maximum number of metastases was exclusionary with size criteria between 5 and 20 mm. Leptomeningeal disease was excluded. Among the melanoma cohort, there were four PRs (95% CI: 7–48) with ongoing response at 4 months when data cutoff occurred. There were two non-responders with SD and eight with progression. ORR was 22%. AEs included grade 1–2 seizure after which all patients were started on antiepileptics. One patient stopped therapy for grade 3 increased aminotransferases, and other reported irAEs were noted to be quite tolerable. In a patient with radiographic progression in all lesions, biopsy revealed only inflammation (66) (NCT02085070).
The ongoing phase II, CheckMate 204, trial using ipilimumab and nivolumab (same dosing regimen as in CheckMate 067) seeks to enroll 110 patients with ≥1 measurable, unirradiated brain lesion(s) measuring 0.5–3.0 cm. The only prior treatments allowed are adjuvant BRAF/MEK inhibition or IFN-alpha and patients with leptomeningeal disease are excluded. Preliminary safety data of 61 patients showed a similar safety profile as in prior studies using the same regimen with 39% of patients experiencing grade 3–5 AEs, no evidence of increased neurological AEs, and with cutaneous and gastrointestinal AEs occurring in >50% of patients (69%, 51%, respectively). There was one treatment-related death from immune-mediated myocarditis. Efficacy data are eagerly awaited (67).
A phase I exploratory study using the same regimen as in CheckMate 067 (ipilimumab and nivolumab versus nivolumab, without an ipilimumab monotherapy arm) for patients with previously untreated brain metastases has shown a similar safety profile as previously seen with this regimen. The ORR of target lesions in the ten patients on each study arm was 50% (95% CI: 19, 81). Median PFS in the nivolumab group has not been reached and was 10.8 months in the combination arm (95% CI: 1.2–). Mediation time to response for the combination versus nivolumab was 11.3 and 12 weeks, respectively, with median DOR still outstanding for both groups (68).
Case 5
A 45-year-old woman presents with a pT3b ulcerated melanoma on her great toe. Lymphoscintigraphy identifies lymphatic drainage to the groin. One sentinel lymph node has a 5-mm focus of invasive melanoma; final stage IIIB (pT3bN1a). PET-CT is negative for other sites of disease. She undergoes inguinal lymph node dissection followed by adjuvant systemic therapy with ipilimumab.
Case in context: adjuvant management of high-risk localized melanoma
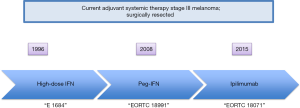
Lymph node metastases confers a risk of recurrence following definitive surgery that is variable by substage. Five-year survival for stage IIIA, IIIB, and IIIC is 78%, 59%, and 40% respectively (P<0.0001) (69). Prior to checkpoint blockade, the only FDA-approved therapies for melanoma in the adjuvant setting were interferon (IFN) and pegylated-interferon (PEG-IFN). In 2015 ipilimumab was approved for use in the adjuvant setting (Figure 3).
IFN exerts immunomodulatory effects via increasing TILs, decreasing regulatory T-cells (Tregs), changes in cytokine concentrations and other signaling and immune regulatory mechanisms (70). The randomized phase III, ECOG 1684, trial comparing high-dose IFN-alpha-2b versus observation demonstrated median PFS from 1 to 1.7 years and OS from 2.8 to 3.8 years. Administration of IFN in this trial was daily for 1 month, then 3 times per week for 48 weeks (71). Therapy is often stopped for significant toxicities including depression (40%), pyrexia (up to 35%), and liver toxicity (up to 29%). Low-dose IFN has been evaluated in the adjuvant setting, but no OS benefit has been demonstrated (72). Pegylated interferon alfa-2b requires less frequent dosing than unpegylated interferon. The phase III, EORTC 18991, trial randomized 1,256 patients to pegylated interferon alfa-2b for 5 years or observation alone to determine long-term efficacy. Patients received pegylated interferon alfa-2b 6 µg/kg a week subcutaneously for 8 weeks (induction phase), followed by 3 µg/kg per week subcutaneously for up to 5 years. After median follow-up of 3.8 years, although fewer recurrences were seen in the treatment versus observation group (N=328 versus 368, respectively), no significant difference was seen in distant metastasis-free survival nor in OS (73).
Data from checkpoint blockade in the adjuvant setting are emerging. Ipilimumab was granted FDA-approval in 2015 for patients with cutaneous melanoma with pathologic involvement of regional lymph nodes of more than 1 mm who have undergone complete resection, including total lymphadenectomy. Approval was based on the EORTC 18071 trial, a randomized, double-blind, placebo-controlled trial in 951 patients with resected melanoma, Stage IIIA (lymph node >1 mm), IIIB, and IIIC (with no in-transit metastases). Ipilimumab was given at 10 mg/kg IV for 4 doses every 3 weeks followed by 10 mg/kg IV every 12 weeks for up to 3 years, a dose higher than that previously approved for metastatic melanoma. Initial data showed a superior relapse-free survival (RFS) of 26 months with ipilimumab compared to 17 months with placebo (18). Updated survival data presented at the European Society of Medical Oncology (ESMO) showed a 5-years OS rate of 65.4% in the ipilimumab group compared to 54.4% in the placebo group (HR for death, 0.72; 95.1% CI, 0.58, 0.88; P=0.001). The rate of distant metastasis-free survival at 5 years was 48.3% in the ipilimumab group, as compared with 38.9% in the placebo group (HR for death or distant metastasis, 0.76; 95.8% CI, 0.64, 0.92; P=0.002). Immune-related adverse events of grade 3 or 4 occurred in 41.6% of the patients in the ipilimumab group and 5 patients (1.1%) died owing to immune-related adverse events (17).
Additional adjuvant trials exploring checkpoint blockade therapies for the treatment of resected high-risk stage III/IV melanoma include SWOG S1404 (pembrolizumab versus investigator-choice ipilimumab 10 mg/kg or IFN), ECOG E1609 (HD-IFN versus ipilimumab 3 mg/kg versus ipilimumab 10 mg/kg), CheckMate 238 (ipilimumab 10 mg/kg versus nivolumab, KeyNote-054 (pembrolizumab versus placebo), and OpACIN (ipilimumab 1 or 3 mg/kg and nivolumab for 12 weeks after surgery or for 6 weeks pre- and 6 weeks post-surgery).
Case 6
A 67-year-old woman had a stage IIIA (pT4aN1a) melanoma 4 years ago removed from her arm. She now presents with three subcutaneous nodules on her chest. Radiographic staging is otherwise negative.
Case in context: management of nodular and/or cutaneous metastases
In this patient with isolated cutaneous metastases, several options exist. Given the low burden of disease and easily accessible lesions on her chest, she is an ideal candidate for intralesional therapy.
The first reported successful intralesional therapy in melanoma was with Bacillus Calmette-Guérin (BCG) vaccine injected into a metastatic bladder lesion resulting in tumor regression (74). Other patients with melanoma responded, however, at least on died from disseminated BCG. A phase III trial (E1673) using intralesional BCG in melanoma failed to show benefit (75). Other intralesional therapies have demonstrated efficacy in clinical trials, some even eliciting a systemic antitumoral response. Among such therapies are intratumoral electroporation of plasmid IL-12 (76), PV-10 (Rose Bengal) (77), Coxsackievirus A21 (78), and SD 101 (79).
The first-in-class injectable oncolytic virus, Talimogene laherparepvec (T-VEC) was granted FDA-approval in 2015, based on results of the phase III, OPTiM, trial (26) comparing intralesional T-VEC with subcutaneous granulocyte macrophage colony-stimulating factor (GM-CSF). The HSV of TVEC is modified for attenuated viral pathogenicity and has been engineered to stimulate expression of the gene encoding human granulocyte macrophage colony-stimulating factor (GM-CSF). This promotes a local immune response capable of inducing tumor cell apoptosis. The first dose of T-VEC is administered at 106 pfu/mL (to seroconvert HSV-seronegative patients) which is followed by 108 pfu/mL 3 weeks after the first dose and then once every 2 weeks. Therapy is generally well tolerated aside from mild fatigue, chills, and pyrexia. Among 436 patients randomly assigned to TVEC (2:1 ratio compared to GCSF), durable response rate (DRR) was 16.3% compared to 2.1% with GM-CSF (P<0.001). The ORR with T-VEC was also higher than with GM-CSF (26.4% versus 5.7%, P<0.001). Median OS was not significantly different with 23.3 versus 18.9 months (HR, 0.79; 95% CI, 0.62 to 1.00; P<0.051) between T-VEC and GM-CSF, respectively. DRR differences were greatest in stage III and M1a disease than in M1b/c. Average time to response was 4.1 months, and more than half of patients experienced ≥25% increase in the size of lesions or appearance of new lesions before achieving a response.
Exciting new intralesional treatments include antigen-specific targeted vaccine (27) and combination regimens with checkpoint blockade and radiation (28,80). T-VEC in combination with ipilimumab was shown to have synergy in a small phase Ib/II study. Among 18 patients with stage IIIB/IV M1c melanoma, the investigator-assessed CR rate was 33%, with PR in 22%, SD in 17%, and an ORR of 56% (95% CI: 31–79%) (81).
Summary
Immune therapy in melanoma has propelled cancer therapy into a new era. Clinical questions remain about predictive biomarkers to identify responders and patients refractory to therapy; sequencing and appropriate combination design therapy; stimulatory means to enhance the therapeutic response; and how to appropriately select life-prolonging treatments against the high toxicity index seen with some of these therapies. Moving forward, tools for screening patients using predictive biomarkers may help in personalizing therapies and reducing toxicities. Although developments in immune therapy against cancer have evolved dramatically since Coley’s toxin of the 20th century, accurate and precise deployment from this new armamentarium is yet to be realized.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marin Feldman Xavier) for the series “Advances on Clinical Immunotherapy” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.38). The series “Advances on Clinical Immunotherapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coley WB. II. Contribution to the Knowledge of Sarcoma. Ann Surg 1891;14:199-220. [Crossref] [PubMed]
- Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther 1994;64:529-64. [Crossref] [PubMed]
- Ichim CV. Revisiting immunosurveillance and immunostimulation: Implications for cancer immunotherapy. J Transl Med 2005;3:8. [Crossref] [PubMed]
- Himmelweit F. The collected papers of Paul Ehrlich. London and New York: Pergamon Press, 1957.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734-6. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2013, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016.
- Siroy AE, Boland GM, Milton DR, et al. Beyond BRAF(V600): clinical mutation panel testing by next-generation sequencing in advanced melanoma. J Invest Dermatol 2015;135:508-15. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cell 2015;161:1681-96. [Crossref] [PubMed]
- Decatur CL, Ong E, Garg N, et al. Driver Mutations in Uveal Melanoma: Associations With Gene Expression Profile and Patient Outcomes. JAMA Ophthalmol 2016;134:728-33. [Crossref] [PubMed]
- Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 1988;319:1676-80. [Crossref] [PubMed]
- Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985;313:1485-92. [Crossref] [PubMed]
- Alva A, Daniels GA, Wong MK, et al. Contemporary experience with high-dose interleukin-2 therapy and impact on survival in patients with metastatic melanoma and metastatic renal cell carcinoma. Cancer Immunol Immunother 2016;65:1533-44. [Crossref] [PubMed]
- Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol 1989;7:445-80. [Crossref] [PubMed]
- O'Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer 2007;110:2614-27. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med 2016;375:1845-55. [Crossref] [PubMed]
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522-30. [Crossref] [PubMed]
- Freeman GJ. Structures of PD-1 with its ligands: sideways and dancing cheek to cheek. Proc Natl Acad Sci U S A 2008;105:10275-6. [Crossref] [PubMed]
- Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 2013;31:4311-8. [Crossref] [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [Crossref] [PubMed]
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. [Crossref] [PubMed]
- Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016;27:559-74. [Crossref] [PubMed]
- Horvat TZ, Adel NG, Dang TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193-8. [Crossref] [PubMed]
- Weber JS, Postow M, Lao CD, et al. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist 2016;21:1230-40. [Crossref] [PubMed]
- Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 2015;33:2780-8. [Crossref] [PubMed]
- Tagliamonte M, Petrizzo A, Tornesello ML, et al. Antigen-specific vaccines for cancer treatment. Hum Vaccin Immunother 2014;10:3332-46. [Crossref] [PubMed]
- Long GV, Dummer R, Ribas A, et al. Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. J Clin Oncol 2016;34:abstr 9568.
- Song X, Zhao Z, Barber B, et al. Overall survival in patients with metastatic melanoma. Curr Med Res Opin 2015;31:987-91. [Crossref] [PubMed]
- Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000;18:158-66. [PubMed]
- FDA, Oncologic Drugs Advisory Committee. Food and Drug Administration Center for Drug Evaluation and Research Briefing Material, 2004.
- Thatcher N, Anderson H, James R, et al. Treatment of metastatic melanoma by 24-hour DTIC infusions and hemibody irradiation. Cancer 1986;57:2103-7. [Crossref] [PubMed]
- Lee SM, Betticher DC, Thatcher N. Melanoma: chemotherapy. Br Med Bull 1995;51:609-30. [Crossref] [PubMed]
- Crosby T, Fish R, Coles B, et al. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev 2000;CD001215 [PubMed]
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451-8. [Crossref] [PubMed]
- Rosenberg SA, Mulé JJ, Spiess PJ, et al. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med 1985;161:1169-88. [Crossref] [PubMed]
- Rosenberg SA, Grimm EA, McGrogan M, et al. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science 1984;223:1412-4. [Crossref] [PubMed]
- Fyfe GA, Fisher RI, Rosenberg SA, et al. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J Clin Oncol 1996;14:2410-1. [PubMed]
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. [PubMed]
- Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005;23:2346-57. [Crossref] [PubMed]
- Besser MJ, Shapira-Frommer R, Itzhaki O, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res 2013;19:4792-800. [Crossref] [PubMed]
- Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res 2010;16:2646-55. [Crossref] [PubMed]
- Goff SL, Dudley ME, Citrin DE, et al. Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J Clin Oncol 2016;34:2389-97. [Crossref] [PubMed]
- Wu R, Forget MA, Chacon J, et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J 2012;18:160-75. [Crossref] [PubMed]
- Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015;33:1889-94. [Crossref] [PubMed]
- U.S. Food & Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival analysis of KEYNOTE-006. J Clin Oncol 2016;34:abstr 9504.
- Long GV, Atkinson V, Cebon JS, et al. Pembrolizumab (pembro) plus ipilimumab (ipi) for advanced melanoma: Results of the KEYNOTE-029 expansion cohort. J Clin Oncol 2016;34:abstr 9506.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Hodi FS, Kluger H, Sznol H, et al. Abstract CT001: Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial. Update from CA909-003 Trial. AM2016-CT001 Published 15 July 2016.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [Crossref] [PubMed]
- Ascierto PA, Demidov LV, Garbe C, et al. Nivolumab (NIVO) safety in patients with advanced melanoma (MEL) who have progressed on or after ipilimumab (IPI): a single-arm, open-label, multicenter, phase II study (CheckMate 172). J Clin Oncol 2016;34:abstr 9526.
- Weber JS, Gibney GT, Sullivan RJ, et al. Survival outcomes of nivolumab (NIVO) given sequentially with ipilimumab (IPI) in patients with advanced melanoma (CheckMate 064). J Clin Oncol 2016;34:abstr 9517.
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Updated results from a phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment-naive patients (pts) with advanced melanoma (MEL) (CheckMate 067). J Clin Oncol 2016;34:abstr 9505.
- Johnston RL, Lutzky J, Chodhry A, et al. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig Dis Sci 2009;54:2538-40. [Crossref] [PubMed]
- Cheng R, Cooper A, Kench J, et al. Ipilimumab-induced toxicities and the gastroenterologist. J Gastroenterol Hepatol 2015;30:657-66. [Crossref] [PubMed]
- Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One 2016;11:e0160221 [Crossref] [PubMed]
- Kourie HR, Klastersky J. Immune checkpoint inhibitors side effects and management. Immunotherapy 2016;8:799-807. [Crossref] [PubMed]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974-82. [Crossref] [PubMed]
- Dadu R, Zobniw C, Diab A. Managing Adverse Events With Immune Checkpoint Agents. Cancer J 2016;22:121-9. [Crossref] [PubMed]
- Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011;117:1687-96. [Crossref] [PubMed]
- Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012;13:459-65. [Crossref] [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Tawbi H. CheckMate 204: Phase II study evaluating the safety of nivolumab plus ipilimumab in patients with advanced melanoma metastatic to the brain. Society for Melanoma Research Congress 2016.
- Haanen J. Efficacy and Safety of Nivolumab (NIVO) Alone or Combined With Ipilimumab (IPI) in Patients With Melanoma (MEL) Metastatic to the Brain in a Phase 1 Study. Society for Melanoma Research Congress, 2016.
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206. [Crossref] [PubMed]
- Ascierto PA, Kirkwood JM. Adjuvant therapy of melanoma with interferon: lessons of the past decade. J Transl Med 2008;6:62. [Crossref] [PubMed]
- Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17. [PubMed]
- Di Trolio R, Simeone E, Di Lorenzo G, et al. The use of interferon in melanoma patients: a systematic review. Cytokine Growth Factor Rev 2015;26:203-12. [Crossref] [PubMed]
- Eggermont AM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 2008;372:117-26. [Crossref] [PubMed]
- Silverstein MJ, DeKernion J, Morton DL. Malignant melanoma metastatic to the bladder. Regression following intratumor injection of BCG vaccine. JAMA 1974;229:688. [Crossref] [PubMed]
- Agarwala SS, Neuberg D, Park Y, et al. Mature results of a phase III randomized trial of bacillus Calmette-Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I-III melanoma (E1673): a trial of the Eastern Oncology Group. Cancer 2004;100:1692-8. [Crossref] [PubMed]
- Daud A, Algazi AP, Ashworth MT, et al. Systemic antitumor effect and clinical response in a phase 2 trial of intratumoral electroporation of plasmid interleukin-12 in patients with advanced melanoma. J Clin Oncol 2014;32:abstr 9025^.
- Agarwala SS, Andtbacka RH, Rice KN, et al. Intralesional rose bengal for treatment of melanoma. J Clin Oncol 2016;34:abstr TPS9600.
- Andtbacka RH, Curti BD, Kaufman H, et al. Final data from CALM: A phase II study of Coxsackievirus A21 (CVA21) oncolytic virus immunotherapy in patients with advanced melanoma. J Clin Oncol 2015;33:abstr 9030.
- Wang S, Campos J, Gallotta M, et al. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci U S A 2016;113:E7240-E7249. [Crossref] [PubMed]
- Agarwala SS. The Role of Intralesional Therapies in Melanoma. Cancer Network, 2016.
- Puzanov I, Milhem MM, Andtbacka RH, et al. Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. J Clin Oncol 2014;32:abstr 9029^.