
Shelterin genes, germ line mutations and chronic lymphocytic leukemia
Telomeres are distinctive DNA-protein structures that cap the ends of linear chromosomes; they are essential to maintain chromosomal integrity and genome stability. Telomeres are composed by tandem repeats of the non-codificante DNA sequence TTAGGG bound by the shelterin complex. It contains six core proteins: telomeric repeat binding factor 1 (TERF1), TERF2, protection of telomeres 1 (POT1), adrenocortical dysplasia homolog (ACD), telomeric repeat-binding factor 2-interacting protein (TERF2IP) and TERF1 interacting nuclear factor 2 (TINF2), which play fundamental roles in telomere protection, chromosomal stability and regulation of telomere length (TL) (1). In addition, the shelterin complex modulates telomerase activity at chromosome ends, recognizes telomeric DNA and remodels it into a t-loop, which protects the 3’ overhang from being recognized as DNA damage. Alterations in the structure and function of any of these proteins may lead to undesirable DNA damage responses that could be associated to a role in tumorigenesis and cancer progression.
Particularly, POT1 has a critical role in protecting the telomere from DNA damage signaling (1). This protein binds specifically to single-stranded telomeric DNA (ssDNA), influencing the maintenance of telomeric DNA by telomerase and protecting the 5’ end of the chromosome. In addition, POT1 acts as a regulator of telomerase-dependent TL and it can help telomere to form D-loop structure to stabilize telomere as well (2). POT1 forms a heterodimer with ACD protein contributing to the t-loop formation (3). Experimental studies support a dual role for ACD acting in telomere protection preventing DNA damage response and telomere maintenance, preserving telomere function (4). This member of the shelterin complex also interacts with ataxia telangiectasia mutated pathway, thus alterations in ACD may stimulate chromosomal instability and large accumulation of mutations leading to cancer development (5). In contrast to the other shelterin components, TERF2IP is not a protective protein, but prevents telomere recombination and fragility. TERF2IP does not bind DNA on its own, but is brought to the telomere by binding to TERF2 (6), and is critical for inhibiting non-homologous end-joining of mammalian telomeres (7).
Chronic lymphocytic leukemia (CLL) is one of the most common types of adult leukemia in Western world. It is also the lymphoid malignancy in which familial aggregation has been described more frequently, showing an eightfold increased risk in first-degree relatives (8). Studies using genome-wide association methodology have contributed to identify several CLL susceptibility loci, providing evidence for a polygenic basis for CLL predisposition (9,10). However, no data of germ-line alleles that might explain this risk had been found. In a recent report, Speedy et al. (11) demonstrated for the first time the existence of germ-line mutations associated with familial CLL. The authors identified seven families with loss-of-function mutations in shelterin genes: four of them in POT1, two in TERF2IP and one in ACD genes, supporting the importance of telomere-associated genes in the pathogenesis of this pathology. The type of mutation was variable: splice acceptor (one family), missense (two pedigrees) and frameshift (one family) for POT1, missense in both families that harbored TERF2IP mutations and splice acceptor in the only one family with ACD mutated gene. It is important to pointing out that germ line mutations in shelterin genes were previously observed in different familial solid tumors (12-17) and in inherited bone marrow failure (18) but, to our knowledge, they had never been found in hematological malignancies (Table 1).
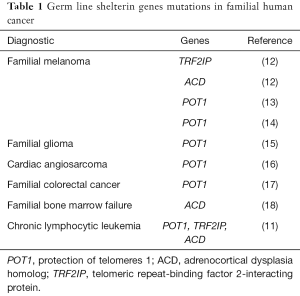
Full table
Interestingly, in the report of Speedy et al. (11) no differences in TL between POT1 mutated and unmutated carriers was found. It is different to those observed in cases of familial melanoma with germ line POT1 mutations (13,14) that showed significantly longer and more fragile telomeres than controls, probably related with the disruption of the interaction between POT1 and ssDNA. The analysis of an increase number of CLL patients with germ line shelterin gene mutations may clarify these discordant findings.
Nevertheless, somatic shelterin gene mutations were described in different lymphoid malignancies. Particularly, Ramsay et al. (19) found somatic POT1 mutations in CLL patients associated to unmutated IGHV (immunoglobulin heavy chain variable region) status, as well as the presence of telomeric and chromosomal abnormalities in the leukemic cells. In line with these findings, POT1 mutations were also observed in cases with cutaneous T-cell lymphomas related to telomere fragility and genome instability (20), suggesting that alterations in this telomere-associated gene could lead to genomic instability and the acquisition of the malignant phenotype. In addition, a somatic ACD mutation was observed in a case of childhood pre-B acute lymphoblastic leukemia (21); in vitro studies support a role for this genomic abnormality in leukemia cells resistance to apoptosis.
A limited number of studies have also evaluated the expression profile of shelterin genes in CLL patients. Among them, Poncet et al. (22) found a global dysregulation of telomere-associated genes, with reduced expression of TERF1, TERF2, TERF2IP and POT1, increased mRNA levels of ACD and no differences for TINF2 as compared to controls. On the contrary, Hoxha et al. (23) using microarray analysis observed TERF1, POT1 and TERF2IP overexpression; these discordant results could be related to the number and clinical characteristics of patients. Furthermore, our group has studied the expression profile of shelterin genes in other lymphoid malignancies. Particularly in mantle cell lymphoma patients, we have observed significant increased expression in all members of the shelterin complex compared to controls (24). In addition, the analysis of cases with plasma cell disorders showed upregulation of shelterin genes in multiple myeloma (MM) patients with respect to cases with monoclonal gammopathy of undetermined significance, with statistical differences for TERF2 and POT1. Particularly, POT1 overexpression was associated with adverse prognostic factors, poor clinical outcome and short overall survival in MM patients, results confirmed by multivariate analysis (25). These data suggested for the first time the participation of POT1 in the process of multistage tumorigenesis in plasma cell disorders and permitted to propose it as a molecular marker in these entities.
Concluding, these new findings in familial CLL patients reported by Speedy et al. (11) add more complexity to the molecular heterogeneity of this pathology, constituting a possible novel prognostic biomarker for cancer patients, and suggest the importance of germ line mutations in telomere-associated genes on cancer-predisposing conditions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Peipei Xu (Department of Hematology, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.02.36). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet 2008;42:301-34. [Crossref] [PubMed]
- He Q, Zeng P, Tan JH, et al. G-quadruplex-mediated regulation of telomere binding protein POT1 gene expression. Biochim Biophys Acta 2014;1840:2222-33.
- O’Connor MS, Safari A, Xin H, et al. A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc Natl Acad Sci U S A 2006;103:11874-9. [Crossref] [PubMed]
- Tejera AM, Stagno d’Alcontres M, Thanasoula M, et al. TPP1 is required for TERT recruitment, telomere elongation during nuclear reprogramming, and normal skin development in mice. Dev Cell 2010;18:775-89. [Crossref] [PubMed]
- Kibe T, Zimmermann M, de Lange T. TPP1 blocks an atr-mediated resection mechanism at telomeres. Mol Cell 2016;61:236-46. [Crossref] [PubMed]
- Kabir S, Sfeir A, de Lange T. Taking apart Rap1: an adaptor protein with telomeric and non-telomeric functions. Cell Cycle 2010;9:4061-7. [Crossref] [PubMed]
- Sarthy J, Bae NS, Scrafford J, et al. Human RAP1 inhibits non-homologous end joining at telomeres. EMBO J 2009;28:3390-9. [Crossref] [PubMed]
- Goldin LR, Björkholm M, Kristinsson SY, et al. Elevated risk of chronic lymphocytic leukemia and other indolent non-Hodgkin's lymphomas among relatives of patients with chronic lymphocytic leukemia. Haematologica 2009;94:647-53. [Crossref] [PubMed]
- Di Bernardo MC, Broderick P, Catovsky D, et al. Common genetic variation contributes significantly to the risk of developing chronic lymphocytic leukemia. Haematologica 2013;98:e23-4. [Crossref] [PubMed]
- Speedy HE, Di Bernardo MC, Sava GP, et al. A genome-wide association study identifies multiple susceptibility loci for chronic lymphocytic leukemia. Nat Genet 2014;46:56-60. [Crossref] [PubMed]
- Speedy HE, Kinnersley B, Chubb D, et al. Germline mutations in shelterin complex genes are associated with familial chronic lymphocytic leukemia. Blood 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Aoude LG, Pritchard AL, Robles-Espinoza CD, et al. Nonsense mutations in the shelterin complex genes ACD and TERF2IP in familial melanoma. J Natl Cancer Inst 2014;107:dju408 [Crossref] [PubMed]
- Robles-Espinoza CD, Harland M, Ramsay AJ, et al. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet 2014;46:478-81. [Crossref] [PubMed]
- Shi J, Yang XR, Ballew B, et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat Genet 2014;46:482-6. [Crossref] [PubMed]
- Bainbridge MN, Armstrong GN, Gramatges MM, et al. Germline mutations in shelterin complex genes are associated with familial glioma. J Natl Cancer Inst 2014;107:384. [Crossref] [PubMed]
- Calvete O, Martinez P, Garcia-Pavia P, et al. A mutation in the POT1 gene is responsible for cardiac angiosarcoma in TP53-negative Li-Fraumeni-like families. Nat Commun 2015;6:8383. [Crossref] [PubMed]
- Chubb D, Broderick P, Dobbins SE, et al. Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nat Commun 2016;7:11883. [Crossref] [PubMed]
- Guo Y, Kartawinata M, Li J, et al. Inherited bone marrow failure associated with germline mutation of ACD, the gene encoding telomere protein TPP1. Blood 2014;124:2767-74. [Crossref] [PubMed]
- Ramsay AJ, Quesada V, Foronda M, et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat Genet 2013;45:526-30. [Crossref] [PubMed]
- Pinzaru AM, Hom RA, Beal A, et al. Telomere replication stress induced by pot1 inactivation accelerates tumorigenesis. Cell Rep 2016;15:2170-84. [Crossref] [PubMed]
- Spinella JF, Cassart P, Garnier N, et al. A novel somatic mutation in ACD induces telomere lengthening and apoptosis resistance in leukemia cells. BMC Cancer 2015;15:621. [Crossref] [PubMed]
- Poncet D, Belleville A. Changes in the expression of telomere maintenance genes suggest global telomere dysfunction in B-chronic lymphocytic leukemia. Blood 2008;111:2388-91. [Crossref] [PubMed]
- Hoxha M, Fabris S, Agnelli L, et al. Relevance of telomere/telomerase system impairment in early stage chronic lymphocytic leukemia. Genes Chromosomes Cancer 2014;53:612-21. [Crossref] [PubMed]
- Panero J, Alves-Paiva RM, Roisman A, et al. Acquired TERT promoter mutations stimulate TERT transcription in mantle cell lymphoma. Am J Hematol 2016;91:481-5. [Crossref] [PubMed]
- Panero J, Stanganelli C, Arbelbide J, et al. Expression profile of shelterin components in plasma cell disorders. Clinical significance of POT1 overexpression. Blood Cells Mol Dis 2014;52:134-9. [Crossref] [PubMed]


