Osimertinib in advanced EGFR T790M-positive non-small-cell lung cancer: the clinical impact of AURA3
Patients with advanced epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC) receive first-line therapy with an EGFR tyrosine kinase inhibitor (TKI). This treatment strategy is based on the results of several phase 3 trials which have demonstrated superiority of EGFR TKIs over first-line chemotherapy in terms of progression-free survival and quality of life in these patients (1). This strategy also mandates EGFR mutation analysis in patients with advanced NSCLC, particularly in those with adenocarcinomas (2). A study in the real-world setting in Central European countries, where lung cancer incidence rates are high, confirmed that EGFR mutation testing has been implemented and that patients with advanced EGFR mutation-positive NSCLC preferentially receive first-line therapy with EGFR TKIs in routine clinical practice (3).
While patients with EGFR mutation-positive NSCLC respond well to first- or second-generation EGFR TKIs, they will eventually develop resistance to these agents after median progression-free survival times of 9–13 months (1). Acquired resistance may be caused by pharmacological changes and molecular changes of the tumor (4,5). Molecular alterations include EGFR target alterations in about 60%, non-EGFR bypass track alterations in about 20% of the patients, histological transformation to small cell lung cancer, epithelial-mesenchymal transformation, and yet to be identified mechanisms (4,5). T790M mutations within exon 20 are the main EGFR target alterations and occur alone in 40–55% and in combination with EGFR amplification in 10% of the patients with acquired resistance. The T790M mutation increases the affinity of EGFR for adenosine triphosphate (ATP), thereby causing resistance to EGFR TKIs (6).
Several treatment options have been studied in EGFR mutation-positive patients with acquired resistance to EGFR TKIs. Treatment decisions have considered slow versus rapid progression, single versus multiple sites of progression, cancer-related symptoms, and other factors. Switch to chemotherapy has been considered as standard treatment. Continuing treatment with TKIs has been considered as an option for patients with only slowly progressing tumors in the absence of symptomatic deterioration. In case of progression at a single site, the addition of local radiotherapy may also be an option. Afatinib combined with cetuximab has also been studied (7,8). The most promising strategy, however, has focused on third-generation EGFR TKIs.
Third-generation TKIs are active against EGFR mutations and the T790M resistance mutation and have only limited efficacy against wild-type EGFR (9,10). Thus these drugs may overcome TKI resistance and result in fewer side effects, particularly in terms of diarrhea and skin rash. Third-generation TKIs that entered clinical development include osimertinib, rociletinib and olmutinib (11-15). While the clinical development of rociletinib and olmutinib have been halted in the meantime due to insufficient efficacy and/or unexpected toxicity, osimertinib has been successfully evaluated in phase 2 and 3 trials in patients with advanced EGFR mutation-positive NSCLC.
Osimertinib
Osimertinib is an oral, irreversible EGFR TKI which is active against both EGFR mutations and the T790M resistance mutation (9,11). It also has activity in the central nervous system, has only little activity against wild-type EGFR and does not bind to the insulin receptor or the insulin-like growth factor receptor (9,11). This favorable drug profile suggested that osimertinib should result in greater clinical efficacy at less toxicity in comparison to first- and second-generation TKIs. Therefore, osimertinib was further evaluated in the AURA program (11,12,15).
In a phase I-II trial in patients who had progressed after pre-treatment with EGFR TKIs, osimertinib resulted in a response rate of 51% (11). The response rate was 61% among patients with T790M mutations and 21% among those without T790M mutations. Median progression-free survival times were 9.6 and 2.8 months in T790M-positive patients and T790M-negative patients, respectively. Adverse events were diarrhea in 47% of patients, rash in 40%, nausea in 22%, and decreased appetite in 21% of the patients. The trial suggested an osimertinib dose of 80 mg once daily for further clinical development.
The AURA2 phase II trial confirmed the efficacy of osimertinib in patients with EGFR T790M mutation-positive NSCLC who had developed resistance to frontline therapy with EGFR TKIs (12). The objective response rate was 70% and the disease control rate was 92%. Median progression-free survival was 8.6 months and median duration of response was 7.8 months.
AURA3
Recently, the results of the AURA3 trial have been published (15). This open-label phase 3 trial compared osimertinib with platinum-based chemotherapy in patients with advanced T790M-positive NSCLC who had disease progression after first-line EGFR TKI therapy. The primary endpoint of the trial was progression-free survival assessed by the investigators. Secondary endpoints included response rate according to investigator assessment, response duration, disease control rate, patient-reported outcomes, overall survival, safety, and side-effect profiles. Patients had to have documented presence of an EGFR mutation and central confirmation of the T790M mutation on the cobas EGFR Mutation Test (Roche Molecular Systems) (15).
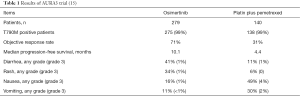
A total of 419 patients with T790M-positive locally advanced or metastatic NSCLC and disease progression after first-line EGFR TKI therapy were randomized in a 2:1 ratio to either osimertinib (80 mg once daily) or pemetrexed (500 mg per square meter of body-surface area) plus either carboplatin (target area under the curve 5) or cisplatin (75 mg per square meter) every three weeks for up to six cycles (15). The major findings of the trial are summarized in Table 1. Patient characteristics were well balanced between the two treatment arms, in particular in terms of age, race, never-smokers, histology, central nervous system metastases, and previous treatments. The distribution of EGFR mutations was similar to those of other studies with exon 19 deletions in about two thirds and L858R mutations in about one third of the patients. T790M mutations were present in 99% of the patients. All patients except one had been pretreated with gefitinib, erlotinib or afatinib.
Patients receiving osimertinib had superior progression-free survival compared to patients treated with chemotherapy (15). The hazard ratio was 0.3 (95% CI, 0.23–0.41) and median progression-free survival times were 10.1 and 4.4 months, respectively. The benefit in progression-free survival was seen across all major subgroups including patients with brain metastases in whom duration of progression-free survival was longer for patients treated with osimertinib than for patients treated with chemotherapy. Patient-reported outcomes were better in patients treated with osimertinib than in patients treated with chemotherapy. The response rate was 71% with osimertinib compared to 31% with chemotherapy. With regard to post-study treatments, 60% of patients in the chemotherapy arm crossed over to osimertinib. Survival data have not been presented yet.
The side effects were different between the two treatment arms (15). Overall, osimertinib was better tolerated as underlined by the lower frequency of grade 3 adverse events compared to chemotherapy (23% vs. 47%). Adverse events more commonly seen with osimertinib were diarrhea, skin toxicity (rash, dry skin, paronychia). Nausea, vomiting, constipation, fatigue, and hematotoxicity were more commonly seen in the chemotherapy arm.
Impact of AURA3
The AURA3 trial demonstrated improved outcome for osimertinib compared to chemotherapy in patients with EGFR mutation-positive NSCLC who had developed T790M-mediated resistance to first- or second-generation EGFR TKIs (15). Improvements with osimertinib over chemotherapy include prolonged progression-free survival, higher response rate and better drug tolerability. Because progression-free survival was the primary endpoint, it was important that improvements in progression-free survival were also accompanied by better patient-reported outcomes. Thus the AURA3 trial established osimertinib as the preferential treatment for patients with EGFR mutation-positive NSCLC at the time of T790M-mediated resistance.
The AURA3 trial was a well-designed phase III trial with progression-free survival as the primary endpoint. The control arm of the trial was adequate as patients received pemetrexed-based chemotherapy. The fact that progression in the AURA3 trial was assessed by investigators is of relevance for the use of osimertinib in the real-world setting in the future when clinicians (and not independent radiological review boards) will monitor treatment response.
The clinical use of osimertinib also requires the determination of T790M mutations in routine practice in either tumor tissues or circulating tumor DNA. The study also proofs that characterization of resistance mechanisms followed by development of drugs to overcome these mechanisms can lead to therapeutic advances. Finally, important questions yet to be answered include the impact of osimertinib on overall survival and the characterization of drugs that will be able to overcome resistance to osimertinib.
Detection of EGFR T790M
The establishment of osimertinib as a treatment for patients with T790M-mediated resistance now also requires proof of the presence of EGFR T790M mutation in tumor cells by either tissue or plasma genotyping. Tissue genotyping remains a clinical challenge because tumor re-biopsy at the time of TKI resistance may be difficult due to poor performance status of the patients and/or limited tumor access. In addition, re-biopsy may also result in insufficient tumor material for genetic analyses. Therefore, minimally invasive plasma genotyping (liquid biopsy) represents an attractive alternative for detection of EGFR T790M. Blood samples are easily obtainable, can be taken repeatedly, and may provide a better picture of the tumor genome than tissue analysis. Furthermore, blood-based analytic approaches also allow real-time monitoring of the total tumor burden and the detection of mutations that will arise during clinical treatment through serial blood sampling and analysis. While the cobas EGFR Mutation Test is currently the only FDA-approved test, highly sensitive digital genotyping assays such as ddPCR or BEAMing can also accurately detect mutations in cell-free plasma DNA (16-19).
Impact of osimertinib on overall survival
The question whether osimertinib also improves overall survival of patients remains to be answered. Until a survival benefit will have been proven, postponing the use of osimertinib after chemotherapy may be considered as an option in selected patients, particularly at times when economic pressure increasingly limits access to novel but expensive drugs. Therefore, the proof of a survival benefit by osimertinib is paramount for establishing osimertinib as the only standard treatment for patients who have developed T790M-mediated resistance to EGFR TKIs.
Resistance to third-generation EGFR TKIs
Patients treated with osimertinib will eventually develop resistance against this drug. Resistance mechanisms that have already been described are EGFR C797S mutations (20,21). A C797S positivity rate of 40% (6 of 15 cases) was recently reported in patients with acquired resistance to osimertinib (21). In cell lines and mouse models, NRAS mutations have been shown to mediate acquired resistance to osimertinib (22). These mutations include NRAS Q61K, E63K, and G12V point mutations as well as a gain of copy number in wild-type NRAS. Whether these molecular alterations are also involved in the osimertinib resistance in patients remains to be determined.
Research on the characterization of drugs that will overcome osimertinib resistance in patients is ongoing. In mouse models, C797S-mediated resistance can be overcome by EAI045 (23). EAI045 targets specific drug-resistant EGFR mutants but spares the wild-type receptor. EAI045 in combination with cetuximab has been shown to be active in EGFR L858R/T790M- and EGFR L858R/T790M/C797S-mutated NSCLC. Although the efficacy of these drugs has yet to be confirmed in clinical trials, these findings are encouraging and further indicate that stepwise improvement in the outcome of treatment with targeted agents has become a clinical reality.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: R.P. has received speaker’s fees from AstraZeneca and Boehringer Ingelheim and honoraria for advisory boards from AstraZeneca, Boehringer Ingelheim and Clovis; M.F. has received speaker’s fees and/or honoraria for advisory boards from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Ratiopharm, and Roche; A.B. declares no conflict of interest.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pirker R. What is the best strategy for targeting EGF receptors in non-small-cell lung cancer? Future Oncol 2015;11:153-67. [Crossref] [PubMed]
- Pirker R, Herth FJ, Kerr KM, et al. Consensus for EGFR mutation testing in non-small cell lung cancer: results from a European workshop. J Thorac Oncol 2010;5:1706-13. [Crossref] [PubMed]
- Ramlau R, Cufer T, Berzinec P, et al. Epidermal growth factor receptor mutation-positive non-small-cell lung cancer in the real-world setting in central Europe: the INSIGHT study. J Thorac Oncol 2015;10:1370-4. [Crossref] [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [Crossref] [PubMed]
- Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473-81. [Crossref] [PubMed]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA 2008;105:2070-5. [Crossref] [PubMed]
- Janjigian YY, Smit EF, Groen HJ, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 2014;4:1036-45. [Crossref] [PubMed]
- Pircher A, Manzl C, Fiegl M, et al. Overcoming resistance to first generation EGFR TKIs with cetuximab in combination with chemotherapy in an EGFR mutated advanced stage NSCLC patient. Lung Cancer 2014;83:408-10. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Walter AO, Sjin RT, Haringsma HJ, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov 2013;3:1404-15. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015;372:1700-9. [Crossref] [PubMed]
- Kim DW, Lee DH, Kang JH, et al. Clinical activity and safety of HM61713, an EGFR-mutant selective inhibi¬tor, in advanced non-small cell lung cancer (NSCLC) patients with EGFR mutations who had received EGFR tyrosine kinase inhibitors (TKIs). J Clin Oncol 2014;32:5s.
- Mok TS, Wu YL, Ahn MJ, et al. osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Taniguchi K, Uchida J, Nishino K, et al. Quantitative detection of EGFR mutations in circulating tumor DNA derived from lung adenocarcinomas. Clin Cancer Res 2011;17:7808-15. [Crossref] [PubMed]
- Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res 2014;20:1698-705. [Crossref] [PubMed]
- Sacher AG, Paweletz C, Dahlberg SE, et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol 2016;2:1014-22. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Ercan D, Choi HG, Yun CH, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res 2015;21:3913-23. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Eberlein CA, Stetson D, Markovets AA, et al. Acquired resistance to the mutant-selective egfr inhibitor azd9291 is associated with increased dependence on ras signaling in preclinical models. Cancer Res 2015;75:2489-500. [Crossref] [PubMed]
- Jia Y, Yun CH, Park E, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016;534:129-32. [Crossref] [PubMed]