
Surgical management of colorectal cancer: the Fudan University Shanghai Cancer Center experience
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide. In China, it is the fifth most common cancer and also the fifth most common cause of cancer-related death. Notably, the incidence and mortality has been constantly decreasing in most developed countries, like the United States, because of early screening and multiple treatment strategies (1,2). However, this trend has not been observed in Chinese patients. The most probable reason for this is the disparity in economic levels between urban and rural areas of China, which leads to unbalanced medical care. Furthermore, public health education requires improvement (3). Therefore, patients with local advanced colorectal cancer or metastasis (mCRC) are still prevalent in China, which presents a great challenge to Chinese oncologists.
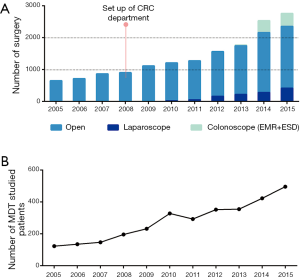
Surgery plays the most important role in CRC treatment. In the recent 5 years, Colorectal Surgery of Fudan University Shanghai Cancer Center (FUSCC) conducted the highest number of CRC surgeries in Shanghai, which reached over 2,000 cases per year. Figure 1A summarizes the number of cases operated in our department. Five-year overall survival rate of patients with localized stage is comparable to most top centers in the world (Table 1).

Full table
Multidisciplinary team (MDT): the cornerstone of CRC treatment
The MDT, including surgeons, physicians, radiologists, radiation therapists, pathologists, and interventional therapists has proved to be very efficient and useful in the management of CRC (4,5). The CRC MDT of FUSCC, established in 2005, is one of the earliest CRC MDTs in China. Difficult cases or late staged cases are recommended for discussion in the MDT meeting each week. Besides, new clinical trials or research should always be presented for suggestion of the MDT members. Figure 1B illustrates the trend of MDT development in the last decade. Because of the excellent job done by the MDT, our center has been selected as one of the five demonstration centers by Ministry of Health of China. Each month, at least 20 doctors visit our center to learn how to run the MDT.
Treatment of precancerous lesion or early staged cancer: role of endoscopic resection
CRC is the second most common cancer in Shanghai. So FOBT with optional colonoscopy is adopted as part of annual physical examination for Shanghai residents, which is financially supported by the Shanghai government. With this change, more and more asymptomatic polyps and early stage cancer be detected.
Once a polyps or neoplasm is detected, biopsy will be performed for pathological diagnosis. Small polyps <1 cm with low grade intraepithelial neoplasia will recommend for loop resection or coagulation. Polyps >1 cm with low grade intraepithelial neoplasia will recommend for endoscopic mucosal resection (EMR). Polyps with high-grade intraepithelial neoplasia and small cancerous lesion <2 cm will firstly underwent endoscopic ultrasound and systematic CT scan, to determine invaded layer, lymph node and distant metastasis. If the lesion is confirmed as locally or early stage, ESD or TEM will be recommended.
Any sample resected by EMR, ESD or TEM will be pin on a wood board and send to pathology for final diagnosis and staging. If a patient was finally diagnosed as early stage CRC, and considered to have unfavorable characteristics, a radical surgery will be recommended. For localization of the resected lesion, titanium clips with abdominal X-ray and methylene blue injection is routinely used pre-operation. The unfavorable features of an early staged cancer include: positive margin, invaded over SM1, neural-vascular invasion, poor differentiation, diameter large than 2 cm. After endoscopic resection, all patients are recommended for endoscopic re-examination at 6 months.
Treatment of locally advanced CRC: precise staging and standardized treatment
Pre-treatment staging
When a patient is diagnosed as CRC, a systematic radiological examination will be arranged. Chest, abdominal, pelvic CT or magnetic resonance imaging (MRI) are routinely recommended for all patients. PET-CT is mostly used in patients with metastasis. However, organ-specific imaging, such as rectum-specific MRI and Pumei (gadoxetic acid disodium injection) liver-specific MRI, are powerful tools for precise staging. It can offer critical information for decision making especially for surgery.
In our center, rectum-specific MRI with diffusion weighted images (DWI) is routinely conducted in all patients with rectum cancer for assessment. Tumor invasion depth (T stage), lymph node status (N stage), extramural vascular invasion (EMVI) and mesorectal fasciae invasion (MRF) and distance from the anal verge should be reported. Coincidence of diagnosis between radiology and pathology in our center is 79.3%. Figure 2 shows different MRI slices of the rectum of one patient before and after neoadjuvant chemoradiation.

Although neoadjuvant chemoradiation is widely accepted as part of the standard treatment for locally advanced rectal cancer and resulted in significant improved local control. Neoadjuvant chemotherapy has not been adopted for locally advanced colon cancer. However, some promising results revealed its role in the future (6,7). Based on contrast-enhanced CT, we define T3–T4 and/or N+ disease as locally advanced colon cancer. A single-arm phase II trial has recently been accomplished in our center. Response rate of neoadjuvant chemotherapy with XELOX was 66% (8). A multicenter randomized Phase III trial lead by our department is now recruiting.
Update of surgical concepts: minimal invasion and function preservation
Minimal invasion and function preservation are two trend of colorectal surgery. There are two ways to make these two trends possible: (I) less resection area. With the use of effective preoperative treatment, tumor shrinkage or down stage is possible. Thus make it a little bit easier for surgeons to achieve satisfied resection margin without resect the adjacent organ; (II) less abdominal wall incision. One of the advantages of laparoscopic surgery is radical resection with minimal incision of the abdominal wall, which would make patient experience less postoperative pain and ileus, and thus accelerate patients recovery. With the development of laparoscopic surgery, more and more clinical trials conclude that laparoscopic surgery is as oncologically safe as open surgery.
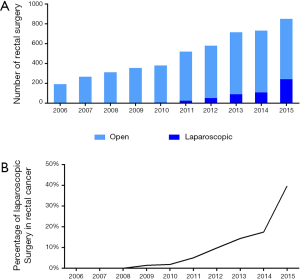
In our center, the proportion of cases operated with laparoscopic approach increased constantly in the latest 5 years. Laparoscopic right/left hemi colectomy, laparoscopic anteria resection, laparoscopic abdominal perineal resection, and laparoscopic total colectomy/colo-rectomy are all our routine surgical procedure. For example, In 2015, altogether 851 patients with rectal cancer underwent resection, in which almost 40% had successfully laparoscopic surgeries (Figure 3A,B). According to the pathological reports, laparoscopic surgery has the same quality of CRF (99.6% vs. 99.6%, laparoscopic vs. open, P=0.920), and lymph node collection (15.9 vs. 15.6, laparoscopic vs. open, P=0.271) compared with open surgery.
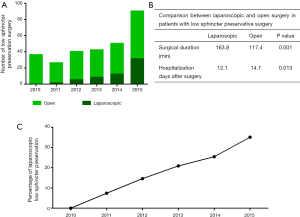
Especially for rectal cancer, advantages of laparoscopic surgery were optimized local views and flexible surgery in the narrow pelvis, which greatly facilitate surgeon to perform better nerve preservation, anal preservation. Development of low sphincter preservation surgeries was summarized in Figure 4A-C. In 2015, 35% of patients with low rectal center underwent laparoscopic sphincter preservation surgeries. The R0 resection rate is 100%. Although surgical duration was longer in laparoscopic approach (P=0.001), hospitalization days are shorter (P=0.013).
Treatment of synchronous mCRC: comprehensive analysis and combined treatment is gradually increasing
Patients with synchronous liver metastasis
Although there have been remarkable improvements in the management of CRC, outcomes remain poor, with approximately 40–50% of patients who undergo curative surgery dying from distant metastases. Liver metastasis is the most common reason for mortality (9-11). The incidence of synchronous liver metastasis, according to Manfredi’s analysis of 13,463 patients with CRC, reached about 14% (12). In patients with colorectal liver metastasis (CLM), radical resection is the only curative therapy (13), which can increase 5-year survival to 50% (14). Patients presenting in our center with synchronous CLM are generally be divided into three groups: those with initially resectable disease; those with potentially resectable disease; and those whose liver metastasis is unresectable.
In the first situation, the question is (I) if we should give neoadjuvant chemotherapy to those with resectable tumors; (II) how to perform the resection, simultaneous or staged? Because the rational for neoadjuvant chemotherapy is to decrease the possibility of recurrence, we routinely use CRS score, which is created by Fong et al. in MSKCC, to evaluate the risk of recurrence. If the score is over 2, then the patient will grouped into high recurrence. Two to three cycle neoadjuvant chemotherapy, usually XELOX will be given to the patient before surgery. We prefer simultaneous resection of primary lesion and CLM as the first treatment choice. Because it could remarkably reduce the number of hospitalization days without increase post-operative complications and influence long-term survival (15,16).
In the second situation, the main question is (I) which regimen we should use for chemotherapy and how to combine with targeted therapy; (II) when to stop chemotherapy and give surgery. All this group of patients will be referred to MDT for discussion, then re-imaging and re-evaluate every 2–3 cycles. If a negative margin and future liver volume rate (FLVR) could be accomplished, surgery, mainly sychronous resection will be performed.
Patients with unresectable liver metastases will be discussed in MDT. Chemotherapy ± target therapy, trans-arterial chemoembolization (TACE), radiofrequency ablation (RFA) were usually given to the patient (17,18). Resection of the primary tumor in mCRC will not be performed if only patients have life-threatening complications, including perforation, bowel obstruction, and severe bleeding.
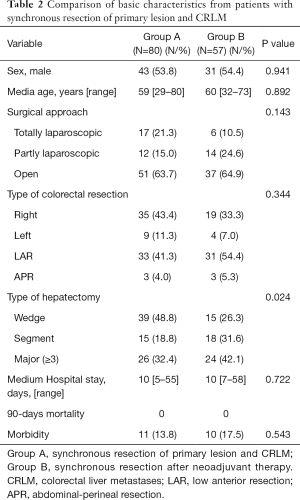
Table 2 demonstrated the safety of simultaneous resection of primary and liver metastases in patients with synchronous CLM in the latest 3 years. Preoperative chemotherapy did not affect the postoperative recovery of patients.
Full table
Patients with peritoneal metastasis (PM)
It had been reported that the incidence of isolated PM ranged from 15% to 25% in patients with stage IV CRC. Patients with PM mostly die from widespread abdominal complications, like bowel obstruction, fistula, or malnutrition. In patients with PM who received no treatment, the median and mean survival was less than 6 months (19). There is increasing evidence supports the surgical management of PM with cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Survival rates could approach 30–45% at 5 years in carefully selected patients, which is similar to the outcomes seen in resected patients with liver metastases (20).
Considering that the sensitivity of imaging examinations remain low, diagnostic laparoscopy with histological confirmation remains the gold standard for evaluating colorectal PM. The peritoneal cancer index (PCI) score is used for measuring the disease burden, while the margin evaluation is used for evaluating the completeness of cytoreduction. Patients with PM in our center, who have a PCI score of less than 20 with good physical status and operable condition, are considered suitable for the cytoreduction with HIPEC. When the intent is curative, HIPEC is applied twice a week.
A clinical trial is now recruiting for patients with tumors at risk of dissemination, like perforation, T4 CRC, or tumor with poor differentiation. The purpose of this trial is to evaluate the usage of HIPEC as a prevention of PM. By now, more than 100 patients have been recruited in this trial.
Extension of routine medical care: management of hereditary CRC
Hereditary CRC, including hereditary nonpolyposis colorectal (HNPCC) and hereditary colorectal polyposis (HCP), comprise about 5–10% of CRC in Chinese patients. Patients with hereditary CRC need individualized treatment strategy. Families with hereditary CRC need genetic counseling for cancer screening and prevention. In 2000, our center developed a diagnostic criterion for HNPCC, which is called the Fudan criteria, based on family history. However, with the decreasing size of Chinese families, diagnostic depending on family history became difficult. Herein, we developed a new routine for the detection and management of hereditary CRC.
HNPCC is the most common hereditary CRC. Most of the HNPCCs are caused by germline mutations in MMR genes, which is also called the Lynch Syndrome (21). Because of inherited MMR gene deficiency, tumors from patients with Lynch syndrome show microsatellite instability (MSI) or loss of expression in one of the MMR gene in immunohistochemistry. In our center, family history should be recorded in all patients. And after surgery, tumor samples of patients should be tested by immunohistochemistry to detect deficiency of MMR. Patients with positive family history or lost express of any of the MMR genes will be suggested for further counseling. Mutation detection of MMR gene should be offered if patient is highly suspected. Fudan Criteria for screening patients with high risks was summarized in Table 3.

Full table
Another comparatively common type of hereditary CRC is familial adenomatous polyposis (FAP). The basic clinical feature of FAP is the numerous polyps spread all over the colorectal, which tend to develop into cancer before age 45. Thus, prophylactic surgery should be considered in all of the FAP patients.
In most cases of hereditary CRC, prophylactic total colectomy proctocolectomy should be considered. After comprehensive analysis of the prognosis of the syndrome and the life quality of the patient, preferred surgical procedure will be discussed with the patient. After surgical resection, intensive surveillance for metachronous cancer should be offered. Colonoscopy will be performed at least once in every 2 years post-surgery. Other organs at high risk should also be assessed during follow-up. First degree relatives should also be included in routine screening.
Conclusions
Precise and individualized standard treatment has become the objective and principle for treatment of CRC MDT in FUSCC. Early detection of precancerous lesions and early cancer call out a challenge for proper selection of patients for endoscopic resection. In CRC, precise diagnosis and staging helps surgeons to make informed decisions. Neoadjuvant therapy and laparoscopic procedure make colorectal surgery less invasive but more possibility for functional preservation. With regard to metastatic CRC, especially liver metastasis, a reasonable therapeutic strategy is the key. Simultaneous resection of synchronous liver metastases is safe. Cytoreduction with HIPEC is a promising treatment for patient with PM. Screening for hereditary CRC is important for affected patient and family. Proper clinical routines are helpful for detection of hereditary CRC.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.09.30). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Burton S, Brown G, Daniels IR, et al. MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer 2006;94:351-7. [Crossref] [PubMed]
- Berho M, Narang R, Van Koughnett JA, et al. Modern multidisciplinary perioperative management of rectal cancer. JAMA Surg 2015;150:260-6. [Crossref] [PubMed]
- Karoui M, Rullier A, Luciani A, et al. Neoadjuvant FOLFOX 4 versus FOLFOX 4 with Cetuximab versus immediate surgery for high-risk stage II and III colon cancers: a multicentre randomised controlled phase II trial--the PRODIGE 22--ECKINOXE trial. BMC Cancer 2015;15:511. [Crossref] [PubMed]
- Arredondo J, Baixauli J, Pastor C, et al. Mid-term oncologic outcome of a novel approach for locally advanced colon cancer with neoadjuvant chemotherapy and surgery. Clin Transl Oncol 2017;19:379-85. [Crossref] [PubMed]
- Liu F, Yang L, Wu Y, et al. CapOX as neoadjuvant chemotherapy for locally advanced operable colon cancer patients: a prospective single-arm phase II trial. Chin J Cancer Res 2016;28:589-97. [Crossref] [PubMed]
- Oliphant R, Nicholson GA, Horgan PG, et al. Contribution of surgical specialization to improved colorectal cancer survival. Br J Surg 2013;100:1388-95. [Crossref] [PubMed]
- Manfredi S, Bouvier AM, Lepage C, et al. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg 2006;93:1115-22. [Crossref] [PubMed]
- Leporrier J, Maurel J, Chiche L, et al. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg 2006;93:465-74. [Crossref] [PubMed]
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. [Crossref] [PubMed]
- Ito K, Govindarajan A, Ito H, et al. Surgical treatment of hepatic colorectal metastasis: evolving role in the setting of improving systemic therapies and ablative treatments in the 21st century. Cancer J 2010;16:103-10. [Crossref] [PubMed]
- Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6; discussion 466-7. [Crossref] [PubMed]
- Yin Z, Liu C, Chen Y, et al. Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): Simultaneous or delayed? Hepatology 2013;57:2346-57. [Crossref] [PubMed]
- Lykoudis PM, O’Reilly D, Nastos K, et al. Systematic review of surgical management of synchronous colorectal liver metastases. Br J Surg 2014;101:605-12. [Crossref] [PubMed]
- Gruber-Rouh T, Naguib NN, Eichler K, et al. Transarterial chemoembolization of unresectable systemic chemotherapy-refractory liver metastases from colorectal cancer: long-term results over a 10-year period. Int J Cancer 2014;134:1225-31. [Crossref] [PubMed]
- Khajanchee YS, Hammill CW, Cassera MA, et al. Hepatic resection vs minimally invasive radiofrequency ablation for the treatment of colorectal liver metastases: a Markov analysis. Arch Surg 2011;146:1416-23. [Crossref] [PubMed]
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [Crossref] [PubMed]
- Elias D, Faron M, Goéré D, et al. A simple tumor load-based nomogram for surgery in patients with colorectal liver and peritoneal metastases. Ann Surg Oncol 2014;21:2052-8. [Crossref] [PubMed]
- Kuiper RP, Vissers LE, Venkatachalam R, et al. Recurrence and variability of germline EPCAM deletions in Lynch syndrome. Hum Mutat 2011;32:407-14. [Crossref] [PubMed]


